Segregation of dopants and defects in ionic materials result in the formation of a space charge zone (SCZ), which is studied extensively for its critical role on functional properties [1]. Although significant theoretical advances have been achieved, the experimental evidence in nanocrystalline ionic materials is indirect. Therefore, we investigated the distribution of defects on the formation of a SCZ in a model system of nano-sized MgO∙nAl2O3 (MAS, n= 0.95 and 1.07). The SCZ was investigated experimentally by electron energy-loss spectroscopy (EELS) and off-axis electron holography (OAEH).
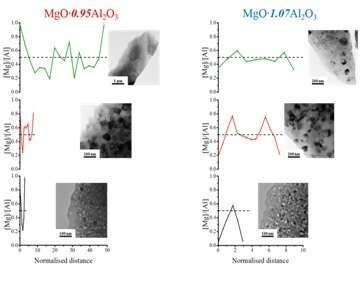
EEL spectra were collected along directions perpendicular to grain boundaries (GB’s), from which the magnesium-to-aluminum relative cation concentrations were calculated, as presented in Fig.1. We found that regardless of annealing processes, the vicinity of GB’s of MgO∙0.95Al2O3 has excess Mg+2 cations while the vicinity of GB’s of MgO∙1.07Al2O3 has excess of Al+3 cations. Additionally, the cation distribution shows strong dependency on the grain size. For non-stoichiometric MAS, cation concentration is proportional to the defect concentration, because deviation from stoichiometry results in adjacent defects that compensate for the electric charge [2, 3, 4]. In both materials, the cation distribution is inhomogeneous for grains smaller than 40 nm. For larger grains, the defect concentration approaches the bulk value at the center of the grain. Furthermore, excess of Mg (Al) cations at the vicinity of the GB decreased with increase of grain size. Maier et al. [1] calculated that for grain size at the scale of the Debye length (estimated at 9nm for non-stoichiometric MAS studied here [7]), the GC is no longer electrically neutral, instead influenced by accumulation or depletion of charge at the boundaries.
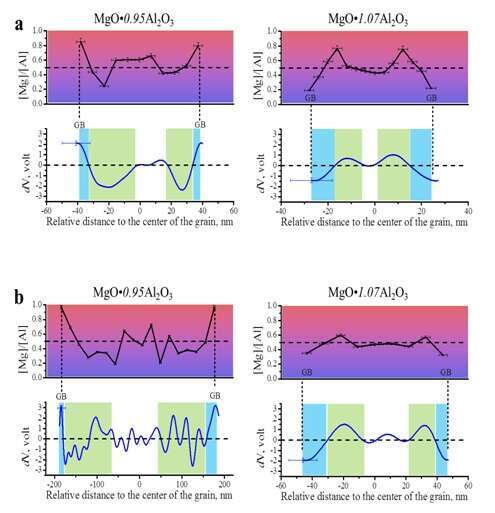
Due to the lack of accurate values for defect formation energy [5, 6], we applied OAEH to measure directly the electrostatic charge distribution in nano-sized MAS. We show that charge distribution and the buildup of electrostatic potential between GB and core are linked to the spatial distribution of defects rather than the overall composition of MAS (Fig. 2). At the vicinity of GB’s, excess Mg+2 or Al+3 cations accumulate depending on the composition, the magnitude of which increases with decreasing grain size. Indeed, the potential distributions show the relation between the grain size and the Debye length, in agreement with theoretical models [1].
Such results underscore the importance of comprehensive nanometer scale characterization of the chemistry and electrostatic potential in ionic complex oxides. Our experimental study enables to verify current models, and more important, develop and test new theoretical models, thus providing a comprehensive understanding of defects in complex oxides.
References:
[1] J. Maier, Prog. Solid State Chem. 23, 171-263 (1995).
[2] M. Rubat du Merac et al., J Am Ceram Soc. 96 (2013) 3341-3365.
[3] S.T. Murphy et al, Philosophical Magazine. 90 (2010) 1297-1305.
[4] Y. Chiang, W.D. Kingery, J Am Ceram Soc. 73 (1990) 1153-1158.
[5] K. Lehovec, J. Chem. Phys. 21, 1123-1128 (1953).
[6] K. Kliewer & J. Koehler,. Phys. Rev. 140, A1226 (1965).
[7] M. Halabi, V. Ezertzky, A. Kohn and S. Hayun, submitted.

