The interaction between amphiphiles and polyelectrolytes has been widely investigated in recent years due to their potential application in industry and medicine, with special focus on gene therapy. The binding of polyelectrolytes to oppositely charged amphiphiles is dominated by electrostatic interactions, but hydrophobic forces and the molecules nature also play an important role; thus influencing the final nano-complex structure.
In this work we used direct-imaging cryo-TEM and SAXS to study the nanostructure of complexes formed in different double-tailed amphiphile/polyelectrolyte systems. The cationic lipid di-oleoyl trimethylammonium propane, DOTAP, and the oppositely charged polyelectrolytes, sodium poly(acrylic acid), NaPAA, and sodium poly(styrene sulfonate), NaPSS, form multilamellar complexes in water. Due to the different molecular stiffness of the two polyelectrolytes, the different morphology of the complexes is distinct. Also, because of different ionization behavior of the two polyelectrolytes, pH affects differently the complexation of the polyelectrolytes with didodecyldimethylammonium bromide (DDAB), a cationic surfactant. PAA demonstrates a pH-dependent behavior, because the carboxylate it has a pKa of 4.2. In contrast, the sulfonate groups, attached to the PSS backbone, disassociate in the entire pH range, hence the complexes formed between the surfactant and the polyelectrolyte are insensitive to pH variations.
In addition, SAXS results demonstrated that the periodic distance in DDAB/NaPSS complexes is almost twice as much as the periodic spacing of DDAB/NaPAA complexes (~6 nm as compared to ~3.3 nm). That is most probably due to the differences in the polyelectrolyte conformation at the surfactant-water interface. Because polystyrene sulfonate is much more hydrophilic than poly(acrylic acid), fully deprotonated at all pH, those charged groups that do not interact with the positively charged headgroups of the surfactant, interact with the water molecules. PAA, with a more hydrophobic backbone, adsorbs more tightly to the surfactant lamellar surface, where it may adopt a flatter conformation, while the more hydrophilic PSS chains interact better with water, and thus may adopt a more coiled conformation, which leads to a thicker layer, and, hence, a larger periodic distance. These differences were not observed in the DOTAP/NaPAA and DOTAP/NaPSS systems, due to a more similar interaction of both polyelectrolytes with the DOTAP head group.
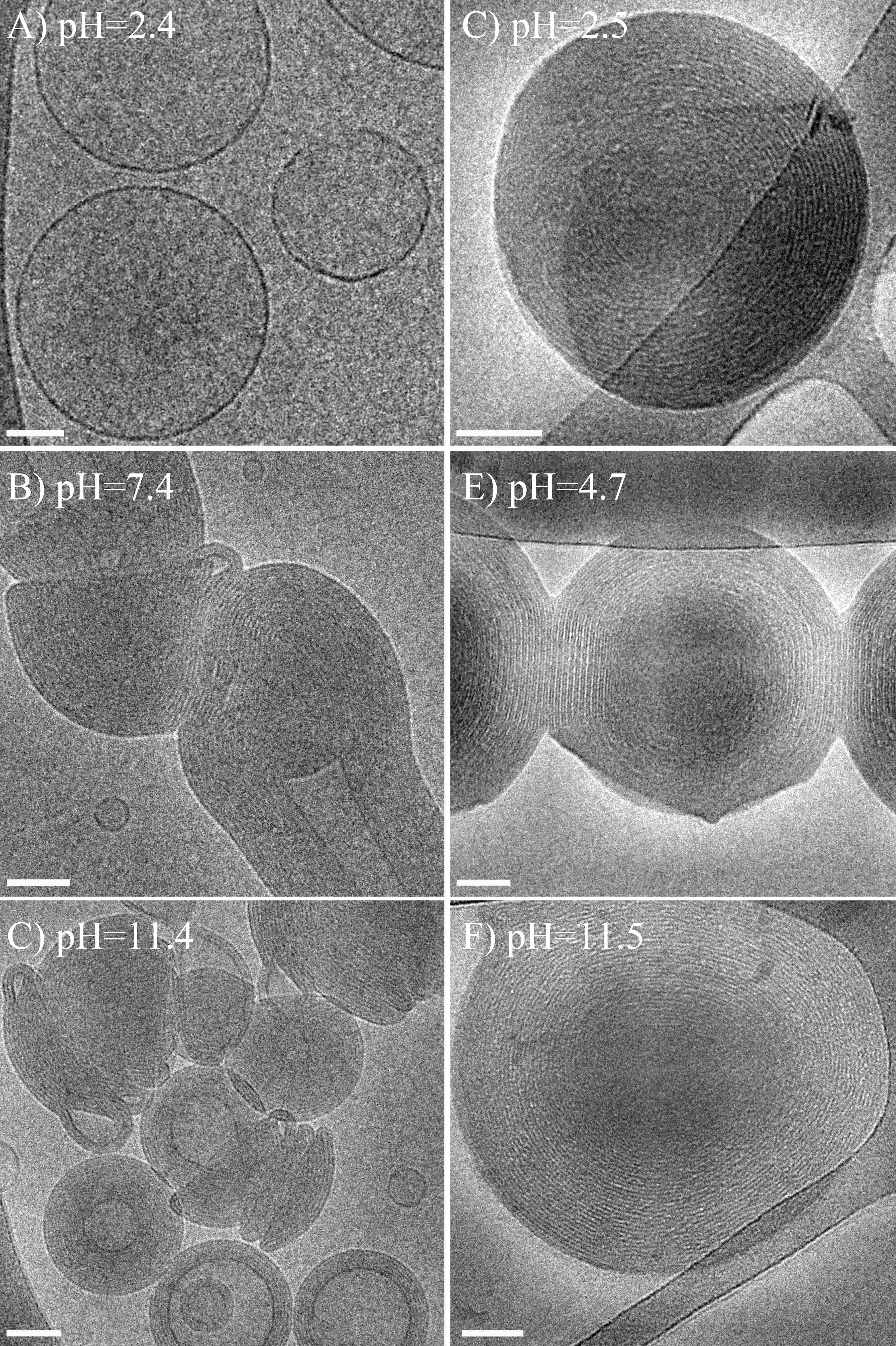
Figure 1. Cryo-TEM images of: DDAB and NaPAA (A-C); DDAB and NaPSS (D-F); CR=1 in all. Bars correspond to 50 nm.

