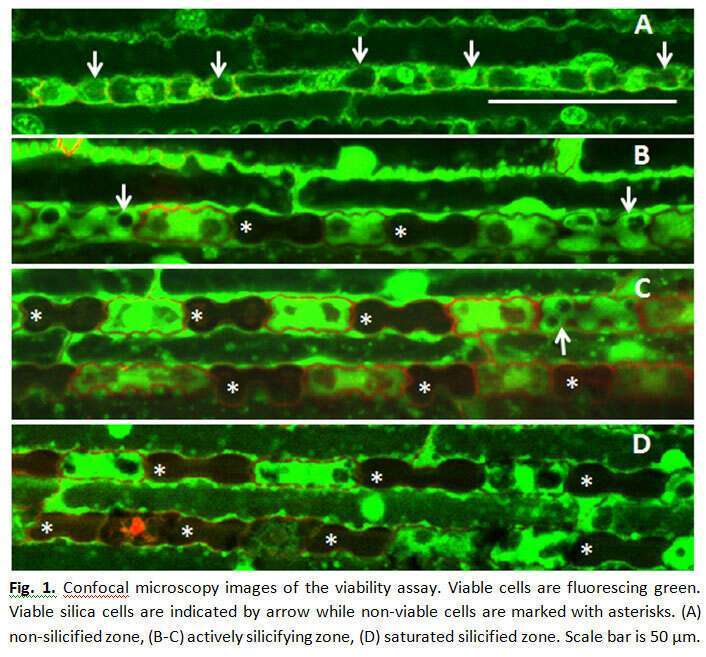
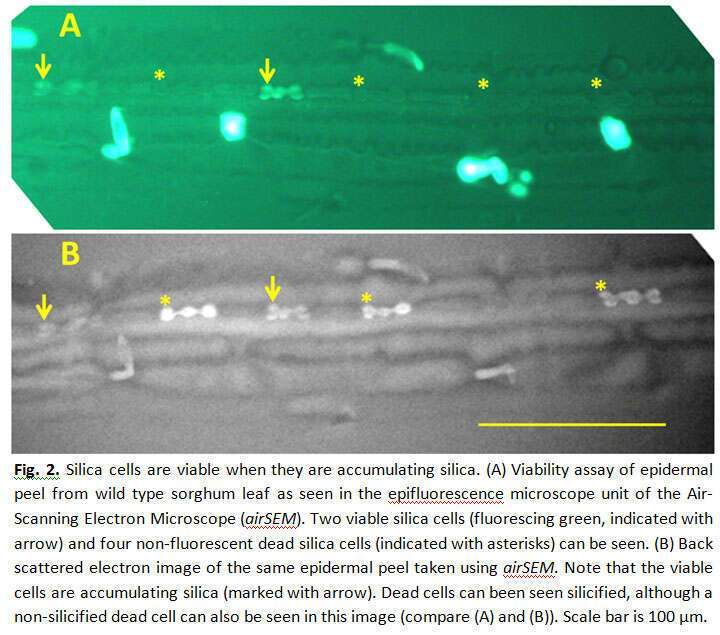
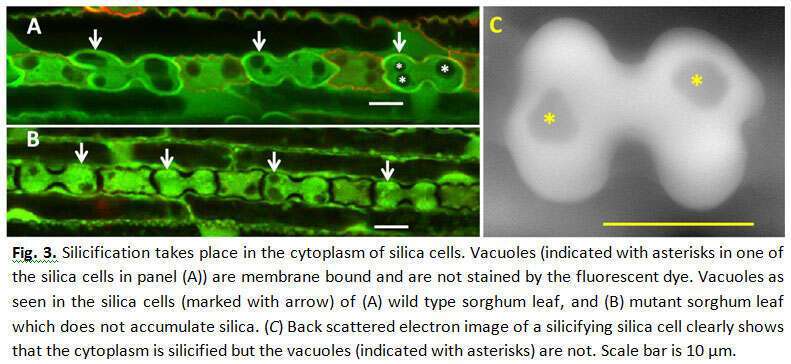
Silicon oxide minerals are major constituents of the earth`s crust, which forms the immediate environment of plant roots. Silica comprises 1-10% of grass dry weight. Soluble silica in the form of silicic acid is taken up by root channels. The silicic acid is then pulled by transpiration into the leaves, and is polymerized into solid amorphous silica in the lumen of specialized epidermal cells called silica cells, and cell walls of other epidermal cells. The mechanism of silica polymerization in grass silica cells in not completely understood. To study the dynamics of silica deposition in leaf silica cells, we identified three zones in rapidly growing young sorghum leaves, namely: non-silicified zone, actively silicifying zone, and saturated silicified zone. We found that silica cells were viable in the non-silicified zone; and non-viable in the saturated silicified zone. Consistent with the variation in the number of silicified silica cells in the actively silicifying zone, silica cells displayed great variation in their viability (Figure 1). Using Air-scanning electron microscopy, in combination with epi-fluorescent microscopy we found that silica cells were viable at the time of accumulating silica (Figure 2). We, thus, were able to answer a long standing question in plant-silicon biology by showing that silicification precedes cell death in silica cells. Interestingly, this unique type of bio-mineralization seems to be taking place in the cytoplasm, unbound by any membrane (Figure 3). The viability of silica cells in sorghum mutant that is unable to uptake silicic acid from soil (0.03% dry weight leaf silicon, as compared to 3.5% in the wild-type) was extended compared to the wild type. We exposed the mutant plants to silicic acid by detaching their roots and supplying silicic acid directly to the shoot. Silica cells were silicified only in young leaf tissues, nicely correlated with their viability. Our confocal observations support the assumption that the mineralization starts from the cell periphery, leaving the cytoplasm within it active and well connected to neighboring cells. As the silicification proceeds, the cell content is petrified, leaving unsilicified voids of vacuoles and vesicles. Our findings show that no transpiration of water is needed for the polymerization, and strongly suggest that silicification is biologically controlled in silica cells.