We report on the synthesis of the newly discovered cubic phase of tin sulfide, π-SnS, and compare its properties to the well- known phase of tin sulfide, α-SnS. This works follows our recent report in which we used Precession Electron Diffraction Tomography (PEDT) technique for obtaining a full structure solution for the π-SnS phase (P213, a0=11.7 Å), a previously unknown simple cubic binary phase of tin mono-sulfide.1 π-SnS polymorph was first identified as tetrahedral nanoparticles present in minute amounts among the reaction products. Selected area electron diffraction (SAED) patterns taken along major zone axes did not conform with α-SnS, the conventional orthorhombic phase of tin sulfide. Yet, all SAED patterns could be fully indexed if a large cubic crystal structure is considered. Due to the small amount of material present in the powder, PEDT technique was the only technique available for full structure solution of π-SnS.
As the research progressed, we were able to improve our synthetic protocol to allow synthesize of both α-SnS and π-SnS separately by controlling reaction parameters.2 It was found that temperature plays a key role in determining the crystallographic structure of the nanoparticles. At higher temperatures (above 200oC) the orthorhombic polymorph of α-SnS appeared, while in milder temperatures (150 oC) the new π-SnS phase was dominant, thus phase control in the synthetic procedure has been demonstrated. Furthermore, shape control was achieved by varying other synthesis parameters, such as the reaction medium and surfactant additives, resulting in cubic, rhombic dodecahedral and tetrahedral shapes of the π-SnS nanoparticles (Figure 1). Shape determination of the cubic and tetrahedral nanoparticles was trivial and did not require further attention. It was found that the cube shaped nanoparticles are formed by exposing (100) facets while the tetrahedral shape is obtained by exposing (111) facets. Regardless, shape determination of the rhombic dodecahedral nanoparticles required thoughtful consideration. In order to realize what is the exact geometrical shape of the particles, a series of HRTEM micrographs were taken with respect to the main crystallographic axes of the nanocrystal. The analysis was based on the relation between the atomic structure of the crystal, its shape and orthogonal projection. Scanning transmission electron microscopy (STEM) was used in order to support the HRTEM data; since the contrast in STEM is mass-thickness, we were able to assign the crystallographic facets coinciding with the nanoparticle surfaces (Figure 2). We conclude that the rhombic dodecahedral shape is obtained by exposing {110} facets, 12 in number.
Recent collaborative work together with the group of K. Nair (UNAM, Mexico) showed that the new π-phase of SnS exists also in thin film form. The films were synthesized in UNAM using chemical bath deposition, and forwarded to BGU for structural analysis. This work was recently submitted for publication jointly with the Nair group.3
Electron energy-loss spectroscopy (EELS) low loss measurements have been carried out in order to monitor possible dielectric function differences between α-SnS and π-SnS particles. This was done by use of Kramers−Kronig analysis in the Digital Micrograph (Gatan) software, which provides an indication for both the real (ε1) and imaginary (ε2) parts of the dielectric function. The results can be seen in Figure 3. A notable difference between the two phases was observed. This signifies that appreciable differences in refractive index and electrical properties between the two nanoparticle types are expected.
We have extended our research to explore the possibility of the existence of analogues structure of π-SnS in the Sn-Se system. SnS and SnSe share much in common, chemically and structurally. Hence, it is natural to question if the selenide analogue of the π-SnS prototype also existed in the form of π-SnSe. In a modified synthesis protocol we have used selenourea as the selenide precursor and investigated the synthesis products. We found that in a similar fashion to the SnS case, the orthorhombic phase was dominant at higher temperatures. At low temperature regime the XRD diffractogram could not be indexed to the orthorhombic phase of SnSe which indicate a different crystal structure. TEM examination of the particles revealed that they appear to be cube shaped. HRTEM of those particles along major zone axes showed net symmetry patterns which could only be fully indexed if a large cubic crystal structure is considered. By adopting the structural model of π-SnS and replacing S atom by Se we established a new model which could account for both XRD results and the HRTEM micrographs. Refining the structural model against the experimental results verified the proposed model in high certainty thus, validating the existence of new binary compound π-SnSe.4
Characterizing the properties of the new π-SnSe phase is currently one of our top priorities. It is in our intention to use EELS in order to evaluate the dielectric function of both α-SnSe and π-SnSe. We speculate that the complex dielectric function might vary similarly to the α-SnS and π-SnS case.
References:
- A. Rabkin, S. Samuha, R. E. Abutbul, V. Ezersky, L. Meshi and Y. Golan, Nano Lett., 2015, 15, 2174–2179.
- R. E. Abutbul, E. Segev, L. Zeiri, V. Ezersky, G. Makov and Y. Golan, RSC Adv., 2016, 6, 5848–5855.
- R. E. Abutbul , A. R. Garcia-Angelmo , P. K. Nair and Y. Golan, “The Puzzle Unraveled: Crystal Structure of Cubic Tin Sulfide in Thin Films”. J. Mater. Chem. A (2016) submitted.
- R. E. Abutbul, E. Segev, Shmuel Samuha, L. Zeiri, V. Ezersky, G. Makov and Y. Golan., A New Nanocrystalline Prototype Structure: Synthesis and Properties of Cubic π-SnSe. CrystEngComm (2016) DOI: 10.1039/C5CE02437D
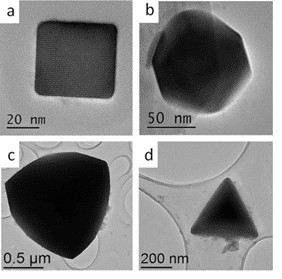
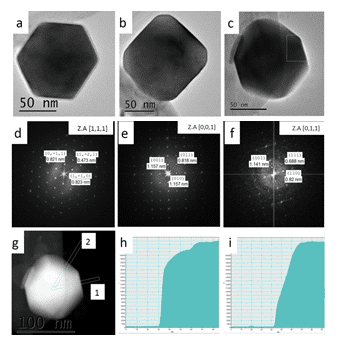
Figure 1.(Left) The four crystal morphologies presented in this work (a) Cubic, exposing (100) facets (b) Rhombic dodecahedral, exposing (110) facets (c) Truncated tetrahedral, exposing combination of (110) and (111) facets (d) Tetrahedral, exposing (111) facets. The dodecahedral, truncated tetrahedral and tetrahedral shapes were obtained using tin-ethylxanthate single precursor with different ODA:OLA ratios. Cubes were obtained using the dual precursor method and were synthesized at 150oC.
Figure 2.(right) π-SnS rhombic dodecahedra shaped nanoparticles viewed at different orientations. (a-c) HRTEM micrographs of select nanoparticles. (d-f) Corresponding FFT micrographs, respectively. (g) STEM micrograph of the nanoparticle seen in (c). (h) Line profile of the selected area labeled “1” in (g). (i) Line profile of the selected area labeled “2” in (g).
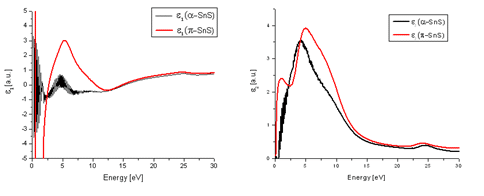
Figure 3. The real (a) and imaginary (b) parts of the dielectric function of both π-SnS and α-SnS. Low loss EELS measurements taken from each nanoparticle type were processed using Kramer-Kronig analysis in order to evaluate the dielectric function.

