 Stomata are small epidermal pores with a complex opening and closing mechanism, which are responsible for the plant gas exchange. Stomata evolved ~400 million years ago - and have remained a key feature of plant anatomy and physiology. Stomata offer a unique research system, where the function has remained largely the same, even though various cell wall features have evolved and changed. We attempted a renewed look at stomatal cell wall structure utilising digitalized polar microscopy and confocal microscopy. We investigated the distribution patterns of cellulose, including microfibril orientation and crystalinity, lignin and phenolic compounds in the stomata of six species of vascular plants. In addition, we applied a numerical mechanical Finite-Element simulation to understand the mechanical anisotropy of the stomatal cell wall. Stomata of the six species chosen for study cover a broad structural, ecophysiological and evolutionary spectrum: two ferns, two angiosperm species with kidney-shaped stomata, and two grass species with dumbbell-shaped stomata.
Stomata are small epidermal pores with a complex opening and closing mechanism, which are responsible for the plant gas exchange. Stomata evolved ~400 million years ago - and have remained a key feature of plant anatomy and physiology. Stomata offer a unique research system, where the function has remained largely the same, even though various cell wall features have evolved and changed. We attempted a renewed look at stomatal cell wall structure utilising digitalized polar microscopy and confocal microscopy. We investigated the distribution patterns of cellulose, including microfibril orientation and crystalinity, lignin and phenolic compounds in the stomata of six species of vascular plants. In addition, we applied a numerical mechanical Finite-Element simulation to understand the mechanical anisotropy of the stomatal cell wall. Stomata of the six species chosen for study cover a broad structural, ecophysiological and evolutionary spectrum: two ferns, two angiosperm species with kidney-shaped stomata, and two grass species with dumbbell-shaped stomata.
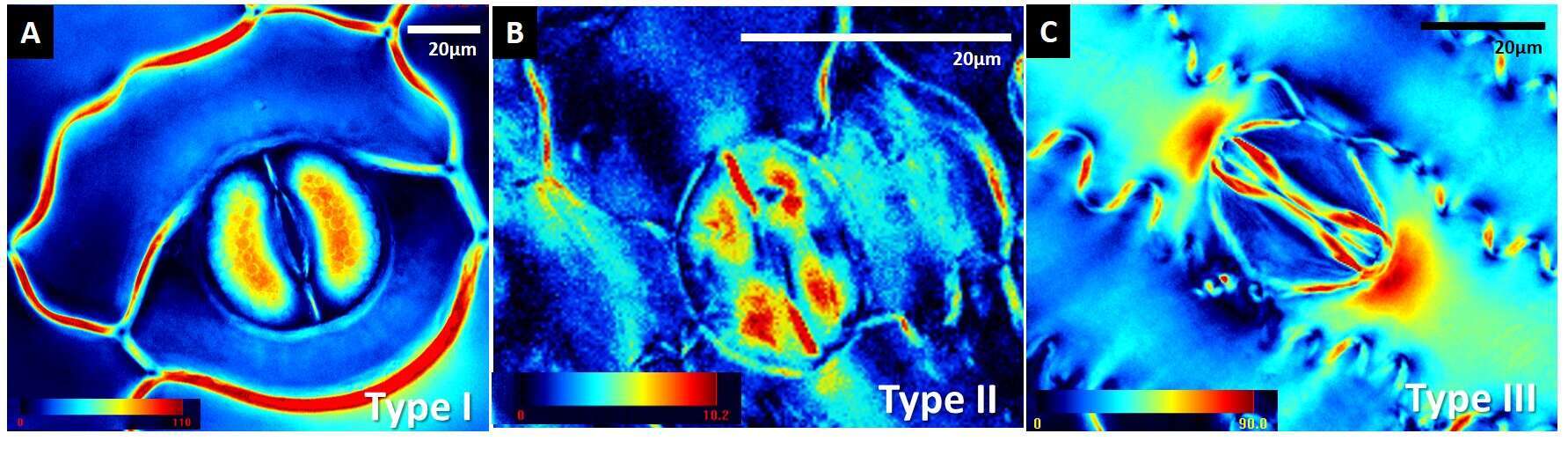
Surprisingly, we observed three distinct patterns of cellulose crystallinity in stomatal cell walls: the ferns exhibited pattern Type I, angiosperm kidney-shaped stomata exhibited pattern Type II and the grasses presented Type III. Our data demonstrates for the first time the existence of distinct spatial patterns of varying cellulose crystallinity in guard cell walls. Guard cell walls undergo reversible deformations during opening/closing of the pore and thus must be both extremely strong and flexible. Different cellulose crystallinity patterns could influence those properties. Such spacial patterns could imply different biomechanical function, which in its turn could be a consequence of different environmental selection. In addition, there were taxon-specific allocation patterns of phenolic compounds and lignin in the guard cells. In ferns the polar end walls were lignified, in angiosperm kidney-shaped stomata the inner (ventral) cell walls contained phenolic compounds and no lignification occurred, and in grasses the whole stoma contained phenolic compounds. According to our numerical bio-mechanical model, the stomatal end walls develop the highest stresses during the opening. It could be an intriguing assumption that crystalline cellulose replaced lignin in stomatal end walls of more evolutionally advanced plants in order to serve a similar wall strengthening function.

