BIOCATALYTIC OXYFUNCTIONALIZATION REACTIONS FOR CHEMISTRY: APPROACHING THE OXYGEN DILEMMA
Oxygenases are the catalysts of choice for specific oxyfunctionalization even of non-activated C-H bonds as they combine reactivity with specificity by reductively activating molecular oxygen in the defined protein scaffold. Their practical applicability, however, is hampered by their cofactor dependency as well as their complicated molecular architecture (Scheme 1).[1]
We have demonstrated that direct reductive regeneration is a viable approach to circumvent the above-mentioned limitations. Thus, highly specific oxyfunctionalization reactions using O2 as oxidant have been achieved.[2] The major challenge observed lies in the high reactivity of intermediate reduced states with molecular oxygen. As a consequence of this ‘oxygen dilemma’, not only valuable reducing equivalents are wasted in a futile cycle but also reactive oxygen species are formed that may impair protein stability.
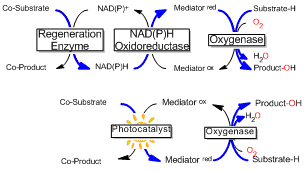
Scheme 1: The simplified direct regeneration approach (lower) compared to the traditional fully enzymatic regeneration (upper).
In this contribution, the nature of the oxygen dilemma will be analyzed and several remedies will be discussed: the oxygen dilemma can be utilized to provide peroxidases with suitable amounts of H2O2 to sustain robust C-H-oxyfunctionalization reactions.[3] Also, the oxygen dilemma can be solved using O2-stable redox mediators to sustain P450-catalyzed hydroxylation reactions. Finally, the oxygen dilemma can be circumvented using O2-independent reductases.
Visible light served as thermodynamic driving force for the abovementioned reactions pointing towards environmentally very benign photoenzymatic oxyfunctionalization procedures. Scope and limitations of this approach will be discussed.
References
Powered by Eventact EMS