OXYGEN REDUCTION AND WATER OXIDATION: PROTON RELAYS, IRON, AND COPPER
Chemistry, University of Washington, Seattle, WA
The electrocatalytic interconversion of dioxygen and water requires management of protons as well as electrons. Proton relays – acid/base sites in the second-coordination sphere of a catalyst – have been shown to be very valuable in hydrogenase catalysis, H2 = 2H+ + 2e–. We have found that proton relays can also substantially improve catalysts for the oxygen reduction reaction (the ORR). The iron porphyrin catalyst with four carboxylic acid proton relays is a rapid electrocatalyst for the ORR in acidic acetonitrile solutions, and is remarkably selective for the four-electron reduction of O2 to water. This is in contrast with the typical formation of substantial hydrogen peroxide by iron porphyrin catalysts. The lack of formation of H2O2 leads to a much longer lived catalyst. The rates and mechanism of ORR by this catalyst, as well as the related tetra(2-pyridyl)porphyrin, will be discussed.
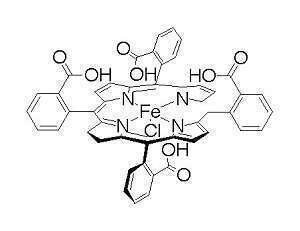
The electrocatalytic oxidation of water by simple copper-bipyridine compounds will also be described. The formation of O2 is observed at high pH, both electrochemically and using a flourescence probe. Electrochemical experiments show that this is a very rapid catalyst for water oxidation, but that it operates at significant overpotentials. The electrochemical results also provide insight into the unusual mechanism for water oxidation.
Powered by Eventact EMS