
Enantioselective Oxidative Homo- and Cross-Coupling of 2-Naphthols Catalyzed by Chiral Iron Phosphate Complexes
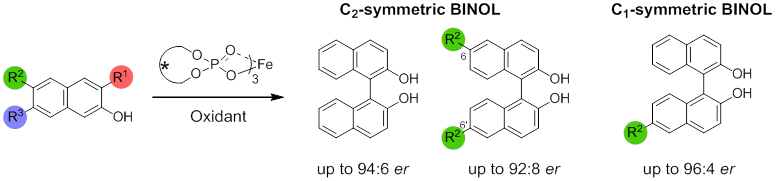
1,1`-bi-2-naphthols (BINOLs) are an important class of compounds. The synthesis of optically pure BINOLs with C1-symmetry generally requires the induction of asymmetry by a chiral catalyst via an oxidative radical-anion coupling mechanism. Asymmetric synthesis of the BINOLs, especially having no substitution at 3, 3` positions is the most challenging process due to competitive oxidative racemization of resulting BINOLs. To address this issue, a novel chiral iron phosphate system has been developed and for the first time successfully applied for synthesis of enantio-enriched C1 and C2 symmetric BINOLs with the 3 and 3` positions available for further chemical modifications. On the basis of kinetic and racemization studies, we postulated the coupling of 2-naphthol involves an intermolecular oxidative radical-anion coupling by iron bisphosphate complex.
References:
- Egami, H.; Katsuki, T. Am. Chem. Soc. 2009, 131, 6082–6083.
- Li, X.; Hewgley, J. B.; Mulrooney, C. A.; Yang, J.; Kozlowski, M. C. Org. Chem. 2003, 68, 5500–5511.
- Libman, A.; Shalit, H.; Vainer, Y.; Narute, S.; Kozuch, S.; Pappo, D. Am. Chem. Soc. 2015, 137, 11453–11460.
- Gaster, E.; Vainer, Y.; Regev, A.; Narute, S.; Sudheendran, K.; Werbeloff, A.; Shalit, H.; Pappo, D. Chem. Int. Ed. 2015, 54, 4198–4202.
- Parnes, R.; Kshirsagar, U. A.; Werbeloff, A.; Regev, C.; Pappo, D. Lett. 2012, 14, 3324-3327.
- Narute, S.; Parnes, R.; Toste, F. D.; Pappo, D. (Communicated)

Powered by Eventact EMS