
Nucleophilic C(sp2)-H Bond Functionalization in Heteroarоmatics as an Atom- and Stage-Efficient Synthetic Tool to Obtain Novel Heterocyclic Derivatives
2Laboratory of Heterocyclic Compounds, Institute of Organic Synthesis, Ekaterinburg
Two principal approaches to modify heteroaromatics by means of incorporation of a nucleophilic fragment through displacement of the C-H bond will be considered.
The first one is based on catalytic activation of the C-H bond. It involves the step of deprotonation followed by the formation of organometallic intermediates, which then react with nucleophiles into the final products (1).
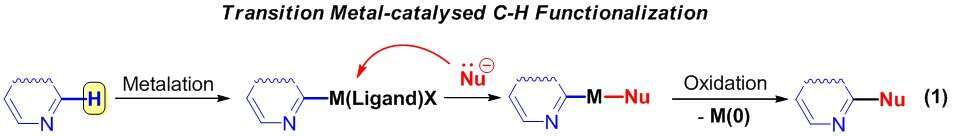
The second approach suggests a direct nucleophilic attack at unsubstituted carbon of an heteroaromatic ring leading to the σH-adducts followed by either two electrons oxidation (“Addition-Oxidation” Protocol 2.1) or elimination of good-leaving group (“Addition-Elimination” Protocol 2.2).1,2
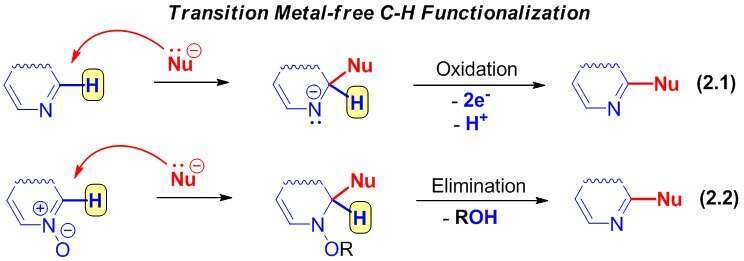
Both schemes involve the departure of proton, however the sequence and the ways of activations differ.
It has been well documented that the metal-free coupling reactions provide a good complementary basis for metal-catalysed cross-coupling reactions, as illustrated by many examples from the chemistry of biologically active compounds, macrocyclic derivatives, polymers, free stable radicals, metallocenes, carboranes, etc.3-7
- Charushin, V.N.; Chupakhin, O.N., Eds. Metal Free C-H Functionalization of Aromatics. Nucleophilic Displacement of Hydrogen. In Top Heterocyclic Chemistry, Maes, B. U. W., Cossy, J., Poland, S., Series Eds.; Springer: Heidelberg, New York, Dordrecht, London, 2014; Vol. 37.
- Chupakhin, O.N.; Charushin, V.N. Tetrahedron Lett. 2016, 57, 2665−2672.
- Varaksin, M.V.; Chupakhin, O.N.; Charushin, V.N. et al. J. Org. Chem. 2012, 77, 9087.
- Varaksin, M.V.; Chupakhin, O.N.; Charushin, V.N. et al. Macroheterocycles 2013, 6, 308.
- Chupakhin, O.N.; Varaksin, M.V. et al. J. Org. Chem. 2009, 74, 2870.
- Utepova, I.A.; Chupakhin, O.N.; Charushin, V.N. et al. J. Org. Chem. 2014, 79, 8659.
- Varaksin, M.V.; Chupakhin, O.N.; Charushin, V.N. et al. Organometallics 2015, 34, 5285.
The study was supported by the RSF (Project № 14-13-01177) and the RFBR (Project № 16-03-00958).

Powered by Eventact EMS