
A Practical Approach to Tunable Oxygen Reduction Catalysts from Mg-Based MOFs
2Schulich Faculty of Chemistry, Technion - Israel Institute of Technology, Haifa
Efficient oxygen reduction holds the key to practical fuel cells and metal-air batteries. Most of today’s oxygen cathodes are based on platinum catalysts. In theory, fuel cells with such cathodes could power cars for mass transportation – but there is simply not enough platinum on Earth to do this.
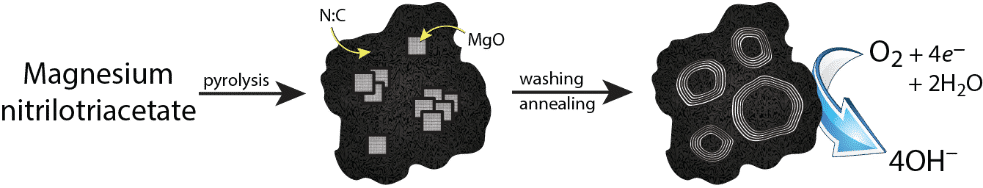
We have discovered a new family of nitrogen-doped carbons, characterized by: (1) a particularly simple synthesis, (2) a complex and fascinating microstructure of the carbon, and (3) very good oxygen reduction reaction (ORR) activity at pH 13.
The material is derived from the pyrolysis of easy-to-make Mg-based MOFs, with or without K+ counterions, and yields a carbon with hierarchical micro/meso/macro porosity. This porosity results from in situ templating by spontaneously forming MgO nanoparticles and from etching by pyrolysis gases. The mesopores are lined with highly graphitic shells. The high ORR activity is attributed to a good balance between high specific surface area and mass transport through the hierarchical porosity, and to improved electronic conductivity through the graphitic shells. Importantly, its synthesis is both cheap and easily scalable.
This elegant carbon synthesis serves as a template for an entire research program in electrochemistry and materials science, whose focus is designability of the microstructure and chemistry. Examples include tuning nitrogen content and pore distribution, and linking it to electrocatalytic activity.

Powered by Eventact EMS