
Carbometalltion/Oxidation of Cyclopropenes: an Oasis of Diastereomerically and Enantiomerically Enriched Cyclopropanols and Aldehydes Possessing Quaternary Carbon Stereocenters
Chemistry, Technion - Israel Institute of Technology, Haifa
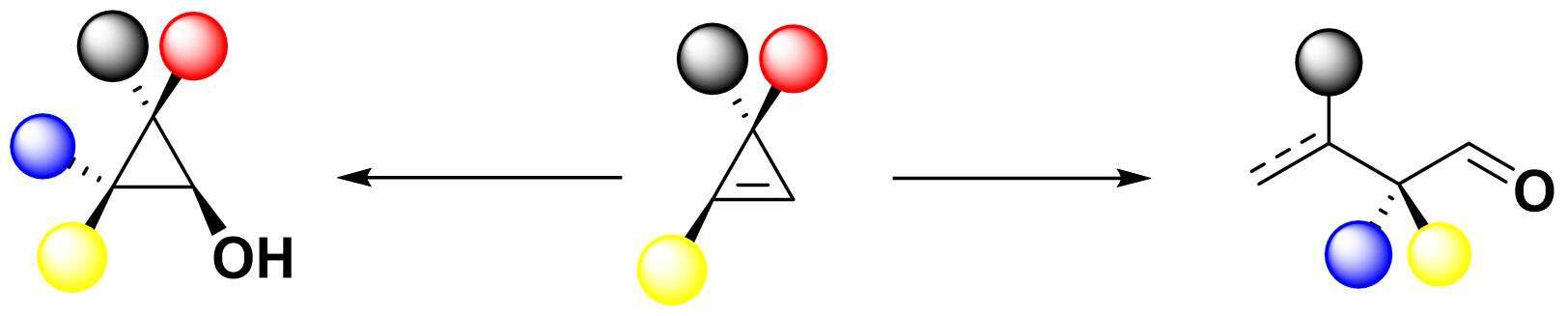
The diastereoselective carbocupration reaction of various cyclopropenes followed by addition of oxenoid
leads to the formation of diastereomerically enriched 2,2,3-trisubstituted and 2,2,3,3-tetrasubstituted cyclopropanol derivatives. Ring fragmentation of the copper cyclopropanolate leads to acyclic aldehydes derivatives possessing a-tertiary and a-quaternary carbon stereocenters in a single-pot operation. The use of enantiomerically enriched starting materails paves the road to the production enantiomerically enriched final products.

Mr. Marwan Simaan
Student
Technion
Powered by Eventact EMS