
Design of Novel Amynoglycoside Derivatives with Enhanced Suppression of Diseases-Causing Nonosense Mutations
New pseudotrisaccharide derivatives of aminoglycosides (AG) that exploit additional interaction on the shallow groove face of the decoding-site rRNA of eukaryotic ribosome were designed, synthesized and biologically evaluated. Novel lead structures (6 and 7 with an additional 7′-OH), exhibiting enhanced specificity to eukaryotic cytoplasmic ribosome, and superior nonsense mutation suppression activity than those of gentamicin, were discovered. The comparative benefit of new leads was demonstrated in four different nonsense DNA-constructs underling the genetic diseases cystic fibrosis, Usher syndrome, and Hurler syndrome.
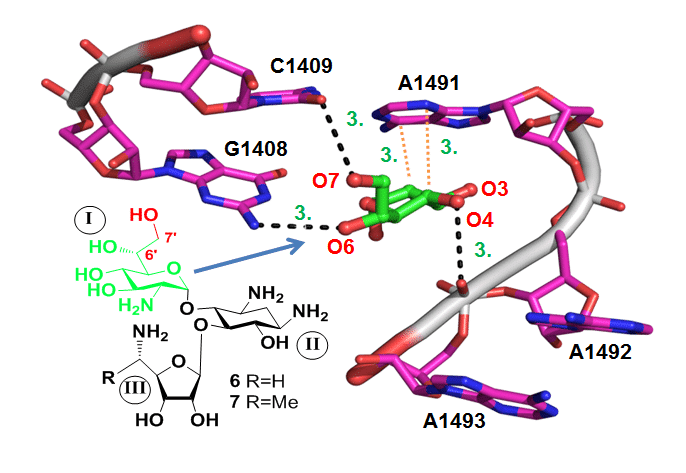
The recently solved X-ray crystal structure of eukaryotic ribosome in complex with the aminoglycoside G418 provided, for the first time, a clear picture of differences between the prokaryotic and eukaryotic sites, which was exploited here for the rational design of new compounds that selectively target the eukaryotic cytoplasmic rRNA A-site. Using this tool, we discovered a new pharmacophore, 7-hydroxyl group, as a valuable structural element of the glucosamine ring that significantly affects eukaryotic versus prokaryotic selectivity. In addition, the observed data support the feasibility of using the rational design strategy employed here for the construction of new AG derivatives that may act as a new drug for the treatment of various genetic disorders.

Powered by Eventact EMS