
Remote Functionalization and Chirality Transfer: Stereoselective Access to Polyfunctional Acyclic Aldehydes bearing Quaternary Centers
The access to stereocontrolled and functionalized quaternary stereocenters is challenging owing to its high steric hinderance.1 Yet, remote functionalization proved to be an efficient strategy to overcome these synthetic issues.2 Based on the combined use of cyclopropylmethanol,3 and Heck reaction,4 we anticipated that the strain release of cyclopropyl moieties would drive the Heck reaction of alcohol 1 towards the formation of the aldehyde 2 (Scheme 1).
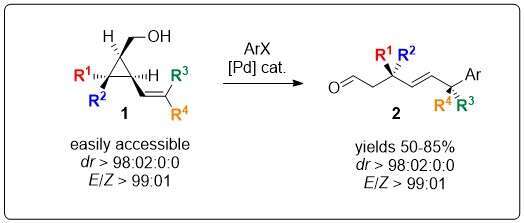
Scheme 1 Access to polyfunctional aldehydes through remote Heck reaction
To our delight the transformation was highly diastereoselective, providing a single (E)-diastereomer. The efficiency of the approach is supported by a straightforward and modular synthesis of vinyl cyclopropylmethanol.5 A representative scope and mechanistic insight will be presented.
1. I. Marek, Y. Minko, M. Pasco, T. Mejuch, N. Gilboa, H. Chechik, J.P. Das, J. Am. Chem. Soc. 2014, 136, 2682.
2. a) J. Bruffaerts, D. Pierrot, I. Marek, Org. Biomol. Chem.2016, 14, 10325; b) A. Vasseur, J. Bruffaerts, I. Marek, Nature Chem. 2016, 8, 209.
3. a) A. Marsawa, D. Didier, T. Zabrodski, M. Schinkel, L. Ackermann, I. Marek, Nature 2015, 505, 199 ; b) A. Vasseur, L. Perrin, O. Eisenstein, I. Marek, Chem. Sci. 2015, 6, 2770.
4. a) E.W. Werner, T.-S. Mei, A.J. Burckle, M.S. Sigman, Science 2012, 338, 1455 ; b) T.-S. Mei, H.H. Patel, M.S. Sigman, Nature 2014, 508, 340.
5. a) D. Didier, P.-O. Delaye, M. Simaan, B. Island, G. Eppe, H. Eijsberg, A. Kleiner, P. Knochel, I. Marek, Chem. Eur. J. 2014, 20, 1038 ; b) D. Müller, I. Marek, J. Am. Chem. Soc. 2015, 137, 15414.

Powered by Eventact EMS