
Synthesis of Sila-Grignard Reagents Via Radical Activation of Si-H Bonds by RMgX or R2Mg
Organomagnesium compounds play an important role in both organic and organometallic chemistry. In contrast, the chemistry of silylmagnesium compounds is very limited, most probably due to the fact that reactions of elemental magnesium with silyl halides do not lead to the formation of silylmagnesium compounds but rather to Wurtz-type silicon-silicon coupling products. Reaction of silyllithium compounds with magnesium halides is currently the most useful method for preparation of silylmagnesium compounds. However the limited number of silyllithium reagents available is a major drawback.
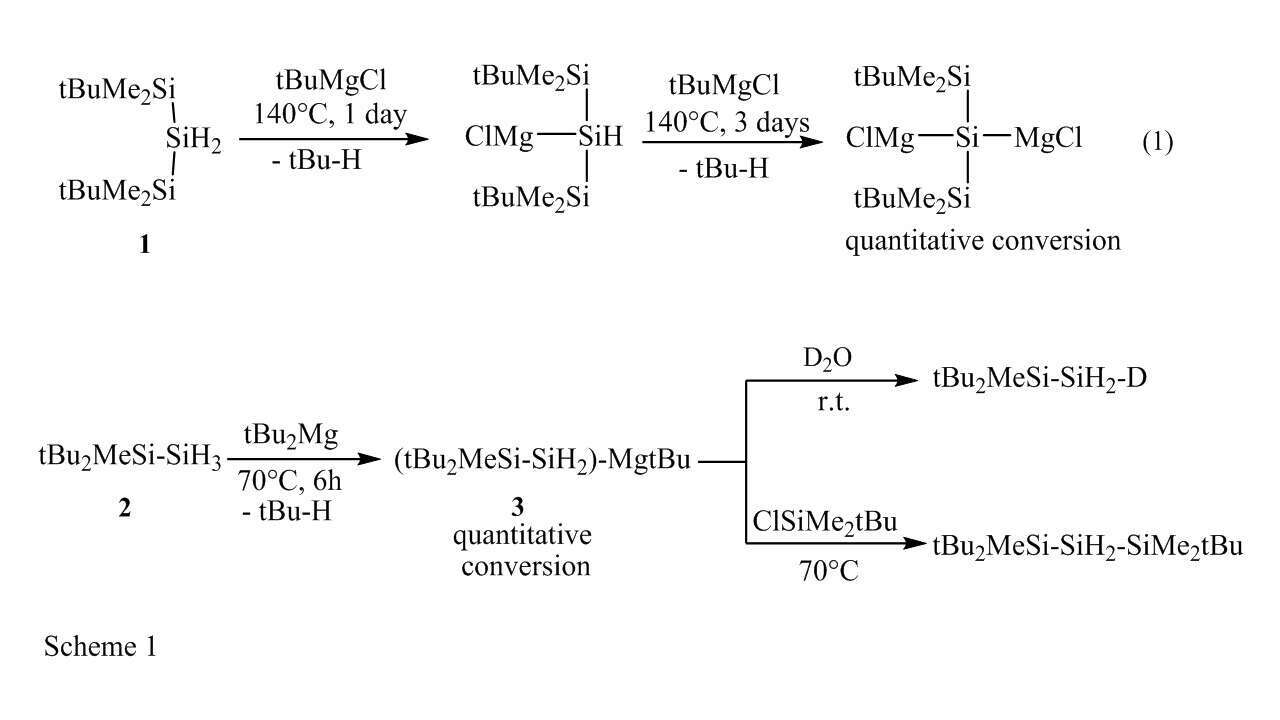
Previously, we reported that organozinc reagents can activate Si-H bonds via a radical mechanism producing silylzinc compounds [1]. Here, we report the first examples of radical activation of Si-H bonds in silyl substituted hydridosilanes R(4-n)SiHn (n=1-3) by tBuMgCl and R`2Mg (R` = nBu, tBu, R``3Si) leading to a direct sila-metalation reaction. Using this reaction we synthesized mono and bis magnesium substituted silanes from the di-hydrido silane 1 (Eq. 1). Moreover, reaction of the tri-hydrido silane 2 with tBu2Mg yields the novel trifunctional mono magnesium silane 3 which was then reacted with several electrophiles (Scheme 1). Addition of tBu2Hg (radical initiator) increased the reaction yield significantly. Addition of 1,4-cyclohexadiene (radical inhibitor) inhibited the reaction completely. These results support a radical mechanism similar to reactions of silanes with organozinc reagents [1].
[1] R. Dobrovetsky, Y. Kratish, B. Tumanskii, M. Botoshansky, D. Bravo-Zhivotovskii, Y. Apeloig, Angew. Chem. Int. Ed. 2012, 51, 4671.

Powered by Eventact EMS