
A Unique Pd–Catalysed Heck Arylation as a Remote Trigger for Cyclopropane Selective Ring–Opening
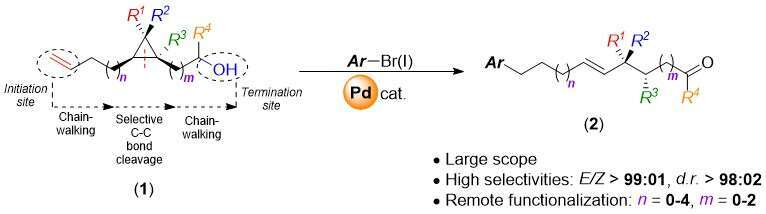
Nowadays, the development of novel organic transformations tends towards more flexible, efficient and economical strategies, as well as the induction of high levels of stereoselectivity. In this context, the concept of remote functionalization has recently drawn great attention as it meets all these criteria.1 For instance, previous studies have identified Heck intermolecular arylations of olefins as a potential remote trigger for distant functionalizations via chain–walking processes.2-6 Hereby, we report the unprecedented Heck regioselective arylation of terminal olefins 1 as a distant trigger for the ring-opening of cyclopropanes. This Pd–catalysed unfolding of the strained cycle, driving force of the chain–walking process, remarkably proved its efficiency and versatility, as the reaction proceeded regardless of the molecular distance between the initiation (double bond) and termination (alcohol) sites in 1. Moreover, employing stereodefined polysubstituted cyclopropane provide access to sophisticated stereoenriched acyclic scaffolds in good yields. Conceptually, we demonstrated that merging catalytically a chain walking process with a selective C–C bond cleavage represents a powerful approach to construct linear skeleton possessing two stereogenic centers.
- Vasseur, A.; Bruffaerts, J., Marek, I. Nature Chem. 2016, 8, 209.
- Molpolder, J. B.; Heck. R. F. A. Org. Chem. 1976, 41, 265.
- Larock, R. C.; Leung, W. Y.; Stoltz–Dunn, S. Tetrahedron Lett. 1989, 30, 6629.
- Werner, E. W.; Mei, T.–S., Burckle, A. J., Sigman, M. S. Science 2012, 338, 1455.
- Mei, T.–S.; Patel, H. H.; Sigman, M. S. Nature 2014, 508, 340.
- Didier, D. et al. Eur. J. 2014, 20, 1038.

Powered by Eventact EMS