
Catalytic Mechanochemistry
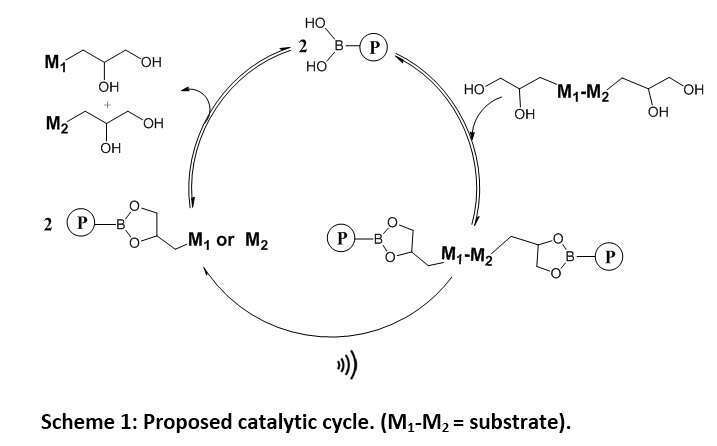
Mechanochemistry is a process in which chemical reactions are driven by mechanical stress;1 however, up to date, mechanochemical transformations were shown to occur only on polymers.2 Importantly, some mechanochemical transformations could be very useful in the synthesis of small molecules such as natural products and drugs, as the mechanical force changes the energy potential of different chemical processes, directing the reaction to different products from the ones obtained by classical thermal, electro and photochemistry. Here, we present an approach to use semi-telechelic polymers capable of reversibly binding small molecule substrates and induce mechanochemical transformations catalytically.
We synthesized boronic ester terminated polymers, capable of binding 1,2- or 1,3-diols reversibly through transesterification reaction. The general catalytic reaction is depicted in Scheme 1. Mechanochemically stable polymers a boronic acid end group (P) and a small molecule (M1-M2) containing two 1,2-diols connect to form a mechanochemically sensitive polymer chain with the substrate at its center (P-M1-M2-P). Under solvodynamic shear, the substrate breaks into two substances (M1 and M2) and is released by trans-esterification with a new substrate.
- Philippe Lavalle, Fouzia Boulmedais, Pierre Schaaf and Loïc Jierry, Langmuir, 2016, 32(29), 7265–7276.
- Jun Li, Jeffrey S. Moore, Chem. Res., 2015, 48, 2181-2190.

Powered by Eventact EMS