
Supramolecular Corroles for the Activation of Small Molecules
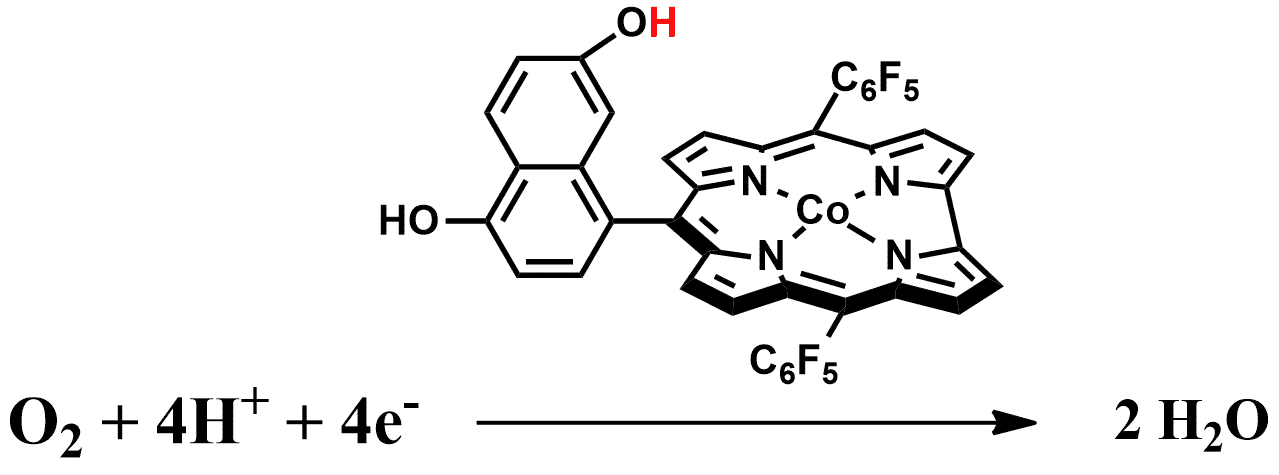
Research on the activation of small molecules such as O2, H2O and CO2 has gained huge impetus in the recent years. The quest for the development of suitable catalysts for oxygen reduction has developed in order to mimic the naturally occurring enzymes such as cytochrome C oxidase. Metallocorroles have been recently investigated to be relevant catalysts for the oxygen reduction reaction because of several intriguing features.1-3 Realizing that the non-sustainable platinum in fuel cells must be replaced, a recent study focused on first row transition metal complexes of β-pyrrole brominated corrole.3 The catalytic activity followed the order of Co> Fe> Ni> Mn> Cu; the onset potential of the Co corrole was almost as positive as platinum; and the selectivity to H2O production rather than H2O2 was quite high.
In order to make the O‒O bond breaking process more organized, an approach involving proton coupled electron transfer has been adopted in specially designed corrole frameworks known as Hangman corroles.1 Our current research is directed towards the development of simple and bio-mimetic Hangman-like motif in the metallocorrole framework and measurement of its catalytic activity/selectivity on oxygen reduction. In particular we wish to compare and comprehend the variation in the catalytic activities upon the introduction of phenolic groups in judiciously designed metallocorrole frameworks.
REFERENCES
(1) Dogutan, D. K.; Stoian, S. A.; McGuire, R.; Schwalbe, M.; Teets, T. S.; Nocera, D. G. J. Am. Chem. Soc. 2011, 133, 131.
(2) Schechter, A.; Stanevsky, M.; Mahammed, A.; Gross, Z. Inorg. Chem. 2012, 51, 22.
(3) Levy, N.; Mahammed, A.; Kosa, M.; Major, D. T.; Gross, Z.; Elbaz, L. Angew. Chem. Int. Ed. 2015, 54, 14080.

Powered by Eventact EMS