
Synthesis of Highly Functionalized Alkenylfluorides by Silver-Mediated Fluorodestannation
2Schulich Faculty of Chemistry, Technion - Israel Institute of Technology, Haifa
The role of fluorine in synthetic and medicinal chemistry receives an ever-increasing attention as fluorine plays a unique role in influencing the conformation, solubility, potency, permeability or degradability of small molecules. The late-stage introduction of fluorine is of great interest as it allows the modification of complex molecules without significantly changing the synthetic route.
In conjunction with our previously reported ruthenium-catalyzed directed trans-hydrostannation of internal alkynes[1], an efficient method for the synthesis of highly elaborate alkenyl fluorides could be implemented (Scheme 1).[2]
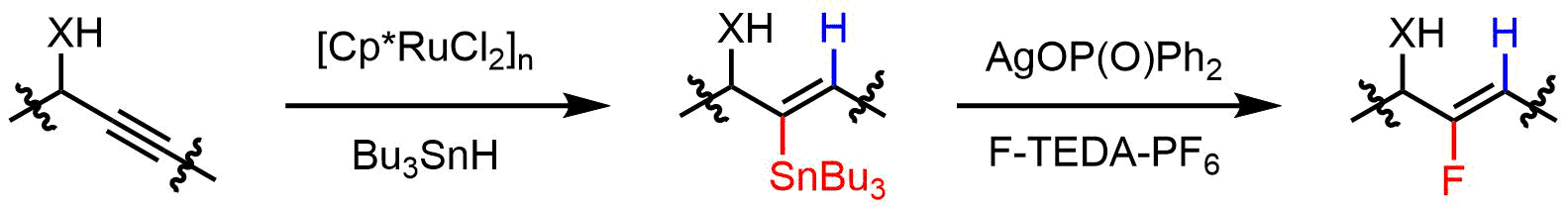
Scheme 1. Hydrostannation/fluorodestannation for the synthesis of fluoroolefins
During our studies, we developed a mild protocol that allowed us to transform a plethora of alkenylstannanes into the corresponding fluorides while overcoming competing protodestannation.[3] Key to success is the utilization of the non-hygroscopic salt silver(I) diphenyl phosphinate (AgDPP) as a mediator.
We applied this new protocol to the synthesis of highly functionalized, biologically relevant compounds, consisting among others of a polyketide derivative, a peptide bioisoster and a prostaglandin derivative (Scheme 2).
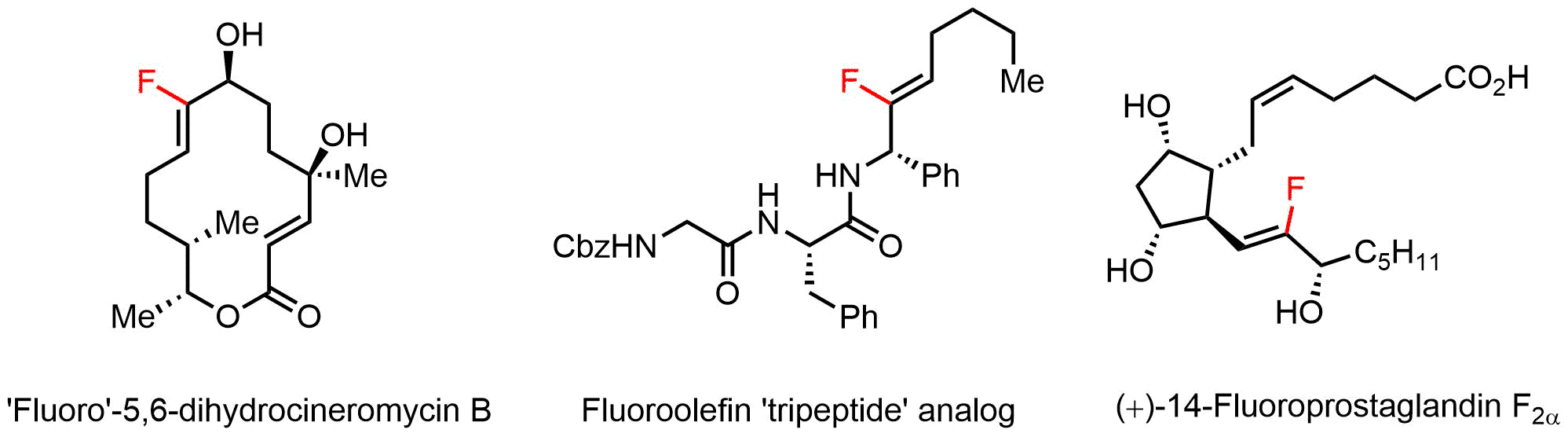
Scheme 2. Selected examples of the silver-mediated fluorodestannation
Literature
[1] a) S. M. Rummelt, A. Fürstner, Angew. Chem. Int. Ed. 2014, 53, 3626-3630; b) S. M. Rummelt, K. Radkowski, D.-A. Roşca, A. Fürstner, J. Am. Chem. Soc. 2015, 137, 5506-5519.
[2] H. Sommer, A. Fürstner, submitted manuscript, 2016.
[3] M. A. Tius, J. K. Kawakami, Tetrahedron 1995, 51, 3997-4010.

Powered by Eventact EMS