
Fluorescent Ruthenium Complexes Based on 2,2’-Bipyridine Modified Peptoids
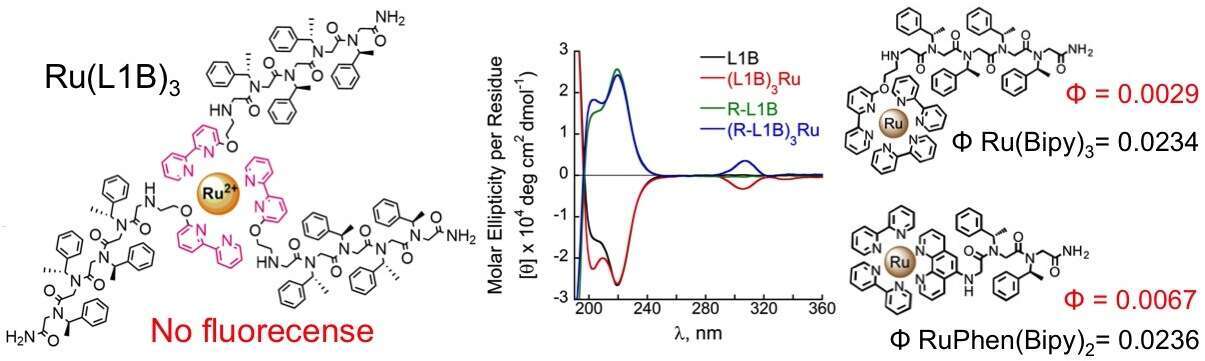
N-substituted glycine oligomers, “peptoids”, are a class of peptidomimetics that are generated from primary amines rather than from amino acids. Thus, their facile and efficient synthesis on solid support enables the incorporation of various functional groups at specified N-positions along their spine. Capitalizing on this property, we designed and generated helical peptoid sequences incorporating 2,2’-bipyridine (bipy), which is a widely used bidentate chelator capable of forming complexes with various metal ions. Herein we demonstrate that such peptoids form Ru(II) complexes via intermolecular binding to three linear peptoid strands or intramolecular binding to a cyclic scaffold. Ru(II) binding promoted changes in the conformational order of the peptoids, and chiral induction from the peptoids to their metal center was observed.1 These Ru(II)peptoid complexes, however, did not show significant fluorescence properties similar to their parent Ru(bipy)3 complex. Our investigations show that a methoxy group bridging between the bipy and the peptoid backbone result quenching of the complexes. Thus, we also describe two approaches to overcome this problem: (1) the use of hereroleptic Ru(II) complexes that include a bipy modified peptoid and bipy or 1,10-phenanthroline (Phen) ligands, and (2) the use of homoleptic Ru(II) complexes with only bipy modified peptoids, in which the methoxy bridge was modified. We demonstrate that these two approaches lead to fluorescent Ru(II)peptoid complexes with quantum yields that are comparable to these of Ru(bipy)3 and Ru(bipy)2Phen complexes.
Reference:
- M. Baskin, L. Panz and G. Maayan, Chem. Commun., 2016, 52, 10350-10353.

Powered by Eventact EMS