
From Frustrated Carbene-Borane Lewis Pairs to Anionic N-Heterocyclic Carbene Ligands
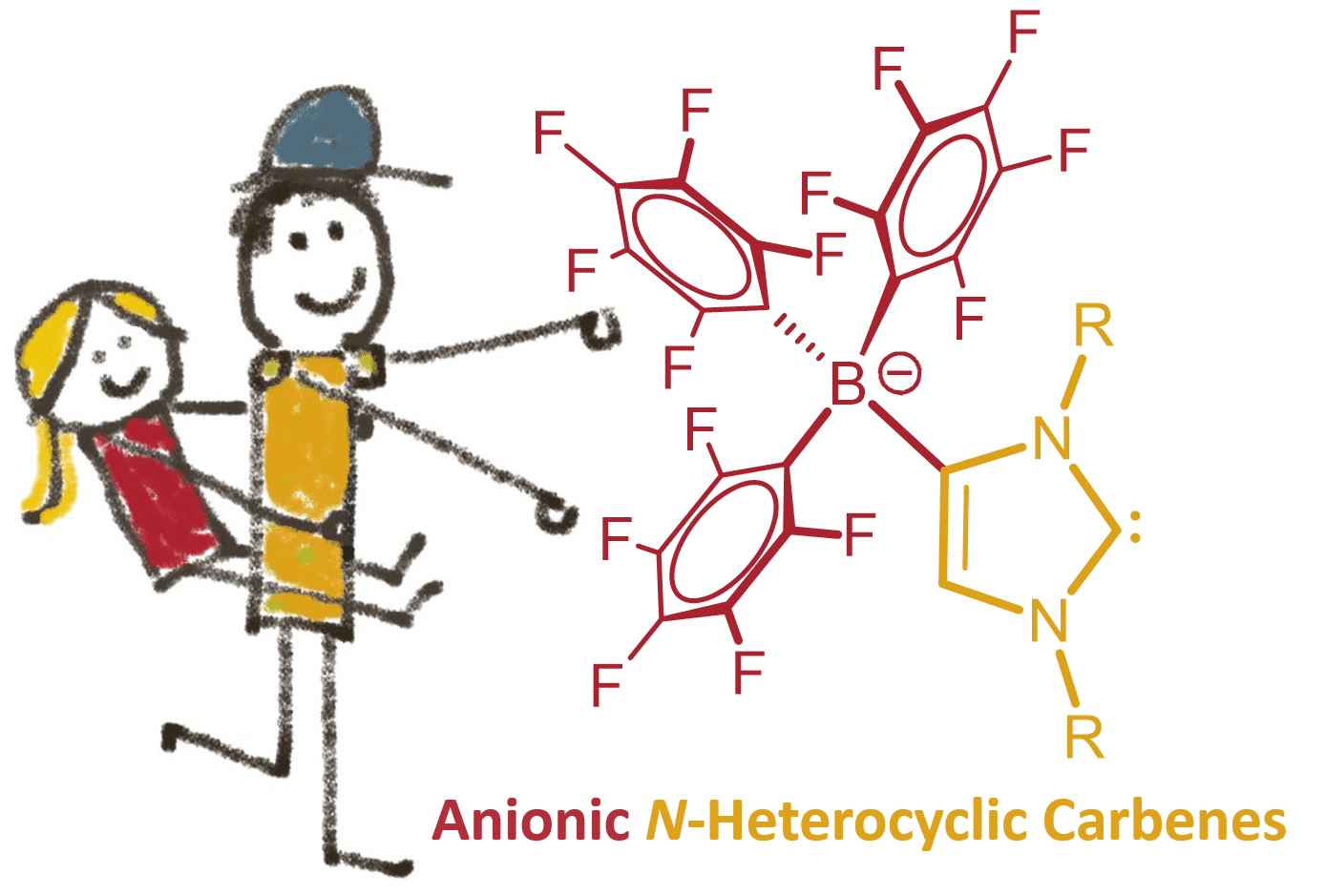
Frustrated carbene-borane Lewis pairs, which are prevented from normal adduct formation by steric factors, were recently shown to exhibit a very strong propensity for the activation of small molecules such as dihydrogen, ammonia, tetrahydrofuran, alkynes, white phosphorus and sulfur [1] In the absence of substrates, however, self-deactivation and irreversible formation of abnormal carbene-borane adducts is observed, which paved the way to the develoment of anionic carbenes bearing a weakly coordinating anionic borate moiety in the backbone (WCA-NHC). These systems proved to be a useful addition to the comparatively small family of anionic N-heterocyclic carbenes (NHCs) [2] and have found useful applications as ancillary ligands in homogeneous catalysis [3-5].
References
[1] Review: “N-Heterocyclic Carbenes in FLP Chemistry”, E. L. Kolychev, E. Theuergarten, M. Tamm, Top. Curr. Chem. 2013, 334, 121-155.
[2] Review: “Anionic N-Heterocyclic Carbenes: Synthesis, Coordination Chemistry and Applications in Homogeneous Catalysis (In memory of Guy Lavigne)”, A. Nasr, A. Winkler, Matthias Tamm, Coord. Chem. Rev. 2016, 316, 68-124.
[3] “Anionic N-Heterocyclic Carbenes That Contain a Weakly Coordinating Borate Moiety”, S. Kronig, E. Theuergarten, C. G. Daniliuc, P. G. Jones, M. Tamm, Angew. Chem. Int. Ed. 2012, 51, 3240-3244.
[4] “Iridium(I) Complexes with Anionic N-Heterocyclic Carbene Ligands as Catalysts for the Hydrogenation of Alkenes in Non-Polar Media”, E. L. Kolychev, S. Kronig, K. Brandhorst, M. Freytag, P. G. Jones, M. Tamm, J. Am. Chem. Soc. 2013, 135, 12448-12459.
[5] “Palladium(II) Complexes with Anionic N-Heterocyclic Carbene–Borate Ligands as Catalysts for the Amination of Aryl Halides”, A. Winkler, K. Brandhorst, M. Freytag, P. G. Jones, M. Tamm, Organometallics 2016, 35, 1160-1169.

Powered by Eventact EMS