
Uridine-5`-O-α,α-Dithiophosphate Analogues: Synthesis and Chemical Properties
Replacement of a phosphate moiety by thiophosphate in nucleotides may be a useful tool for altering binding properties of nucleotides with specific proteins with a view to studying those proteins or developing biologically active molecules. Therefore, the presence of a di-thiophosphate moiety instead of a thiophosphate group is expected to have a unique influence. In this study, a series of uridine-based nucleotides (1-4) were synthesized via a dithiaphospholane intermediate (5). The synthesis was performed by coupling uridine-2’,3’-methoxymethylidene with 3,1,2-dithiaphospholane, followed by phosphorus oxidation with S8 and finally, opening of dithiaphospholane ring by DBU mediated nucleophilic attack by monophosphate, pyrophosphate, or methylene-diphosphonate. The chemical stability of the analogues was evaluated by 31P-NMR regarding air oxidation, as well, as acidic and basic conditions. Most of the analogues exhibited exceptional stability. For instance, analogues 2-4 were stable more than one month under air-flow at RT and showed no disulfide bond formation. Analogues 2 and 3 were highly stable at pH 1.6 for at least 1 week at RT. Analogue 3 exhibited limited decomposition after 25 days at pH 12 (23% of decomposition). The ability of derivatives 1-4 to coordinate physiologically relevant metal ions was studied. Specifically, we titrated analogues 2-4 with Zn2+ and Ca2+-ions. Analogues 2 and 4 bind Zn2+-ion via phosphates α and β and show selectivity toward Zn2+-ions vs. Ca2+-ions, as determined by 31P-NMR. The unexpectedly stable uridine-dithiophosphate analogues may prove useful tools for studying uridine-binding proteins, in particular Zn2+-containing proteins.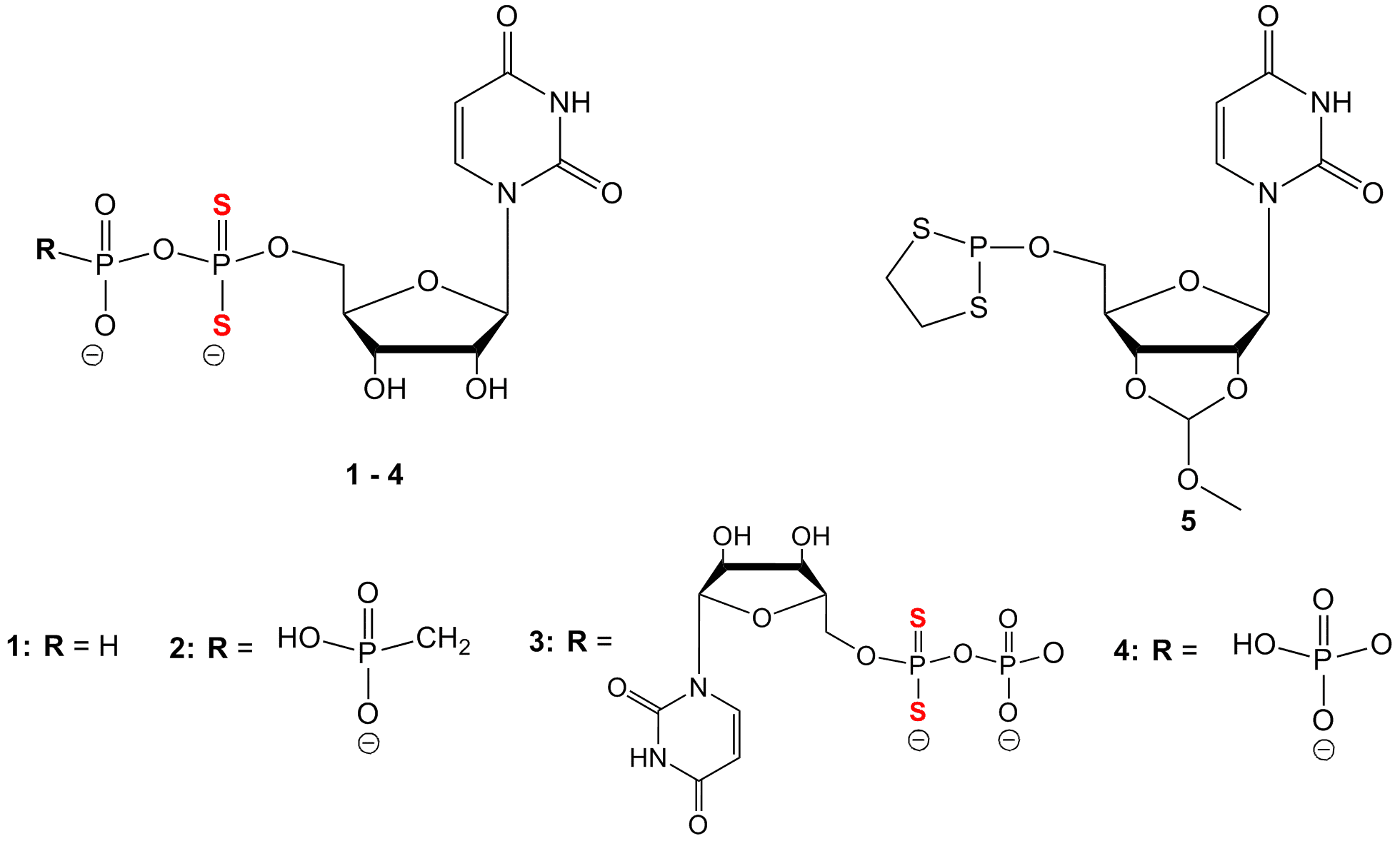

Powered by Eventact EMS