
Anodic Degradation of Amide Bonds
The amide bond is one of the most common bonds in nature, and is known to be strong and stable thermodynamically. Typically, the anodic oxidation of amides affords a side-chain oxidation-substitution product, at the a position to `N` [1], leaving the amide moiety intact:
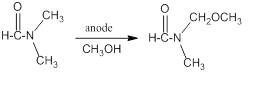
The present work describes that specific amides, lacking hydrogen(s) at the a position to `N`,
undergo three types (A, B and C below) of anodic bond cleavage, in acetonitrile [2].
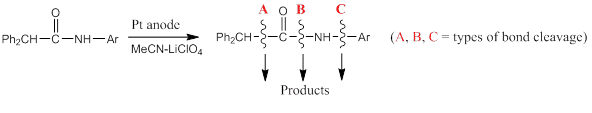
The selectivity of the cleavage and nature of emerged products is highly dependent on the nature of substituent attached to the aryl group. The type of products obtained and the mechanism involved will be discussed, as well as the outcome from `cyclic amides` [3].
References
[1] H. Lund and M. Baizer (Eds.), "Organic electrochemistry: an introduction and guide", 3rd ed., Marcel Dekker Inc, 1990, pp. 1550.
[2] T. Golub and J.Y. Becker, Org. Biomol. Chem., 2012, 10, 3906.
[3] T. Golub, J. Y. Becker, J. Electrochem. Soc., 2013, 160 (7), G3123.

Powered by Eventact EMS