
Overcoming the Lack of Stereocomplementarity within Ene-reductases: The Chemoenzymatic Synthesis of all Four Stereoisomers of 2-Methylbutane-1,3-diol
2Institute of Bio - and Geosciences, Research Center Jülich, Jülich
The chemoenzymatic synthesis of small molecules can provide perfect stereoselectivity where organic methods only supply a certain level of enantiopurity. However, enantiocomplementary enzymes are not always accessible, preventing chemoenzymatic synthesis from becoming a versatile tool in accessing all stereoisomers of a desired product. [1]
Herein we present an approach to overcome this problem by dexterous substrate-design instead of exhausting catalyst-engineering. Two different substrates converted by the same ene-reductase enable access to enantiocomplementary products. Next to the original substrate, ‘mirrored’ starting material can be converted in a similar stereospecific fashion. Afterwards chemical modification of the residues following the enzymatic reaction causes a priority-switch of the residues granting access to the missing isomers.
All remaining stereogenic information is installed by ADHs, matching the advantages of classic organic methods and biocatalysis within a truly chemoenzymatic synthesis to all possible stereoisomers perfectly.
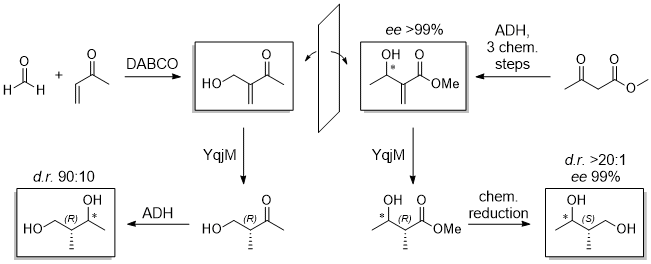
[1] (a) E. Rüthlein, T. Classen, L. Dobnikar, M. Schölzel, J. Pietruszka Adv. Synth. Catal. 2015, 375, 1775-1786 (b) Enzyme Catalysis in Organic Synthesis, Vol. 1, Wiley-VCH Verlag & Co. KGaA, Weinheim, Germany, 2012.

Powered by Eventact EMS