
Metal-free Catalytic Chlorination of Silanes and Silylation of Ethers
Silyl chlorides play an important role in organic synthesis, they are used to protect hydroxyl functional groups. They are also important precursors in organosilicon chemistry and are used for the synthesis of branched organosilanes and sol-gel materials. Various procedures were developed for chlorination of silanes, however, most of these methods suffer from poor selectivity and the use of toxic materials. Herein we present a new selective method for Lewis acid (B(C6F5)3) catalyzed chlorination of silanes by HCl. In addition, we developed a B(C6F5)3 catalyzed method for activation of ethers in presence of silanes, forming silylethers and corresponding alkanes. This method can be potentially used to replace a very robust alkyl ethereal protecting groups to more labile silyl protecting group, this chemistry is currently under investigation. Detailed protocols, the most recent results and the mechanisms supported both by experiment and by theoretical calculations are shown.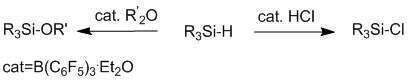

Powered by Eventact EMS