
Adsorption under Nano-Confinement: Predicting Remarkably Higher Levels of Surface Coverage
Remarkable Nano-Confinement Entropic effects on equilibrated Adsorption in a system consisting of small numbers of molecules and adsorption-sites inside a nanoscale space are revealed for the first time by employing statistical-mechanical principles in the combined framework of the ideal-gas and lattice-gas models. The novel effect (termed NCEA) is reflected in computed adsorption isotherms exhibiting considerable positive deviations from the classical Langmuir isotherm of macroscopic systems, namely a system-size dependent significant increase of coverage (Fig.1). Likewise, nano-confined coverage variations with temperature at constant volume exhibit heightened adsorption levels. In particular, the NCEA is found to induce selectivity enhancement for H2O vs. CO as well as for CO2 vs. N2, when the respective two molecular species are co-physisorbed on N-doped carbon materials. Similarly to nano-confinement effects on chemical-equilibrium reported by us previously [1,2], the NCEA origin is associated with distinct intrinsic variations in mixing-entropy and pressure. The present introduction of nano-confined adsorption phenomena indicates the emergence of a new subdiscipline of nanoscience, which can be pertinent also to nanotechnological applications such as gas storage and gas-mixture separation.
[1] "Nanochemical equilibrium involving a small number of molecules: A prediction of a distinct confinement effect" M. Polak, L. Rubinovich - Nano Letters 2008.
[2] "The intrinsic role of nanoconfinement in chemical equilibrium: Evidence from DNA hybridization" L. Rubinovich, M. Polak - Nano Letters 2013.
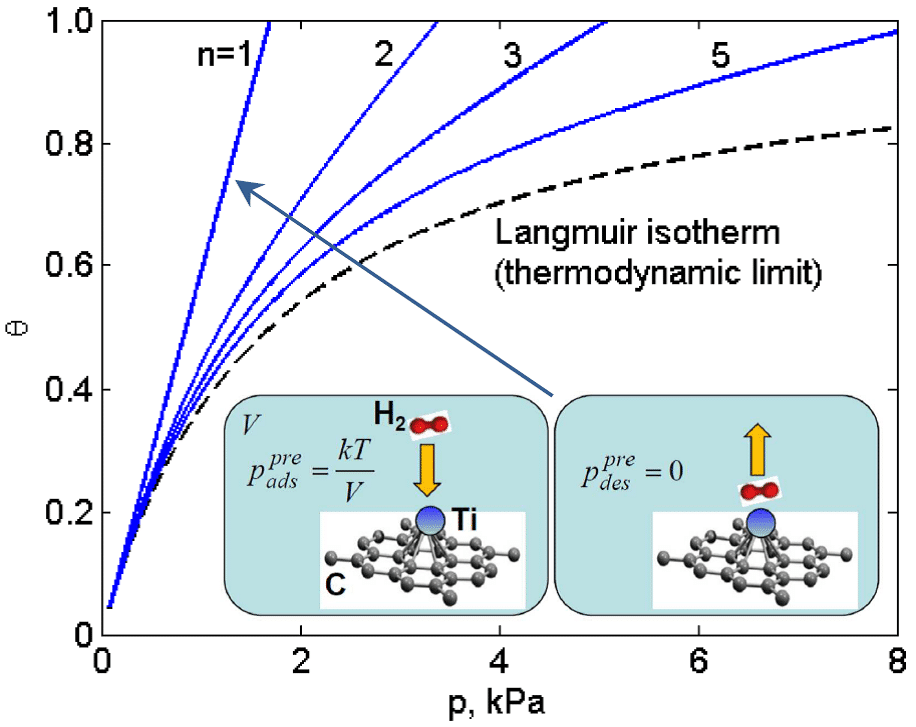
Figure 1. Isotherms at 300 K for n molecules of H2 physisorbed on n Ti-sites in doped graphene under nanoconfinement (varying volume V). Insets: illustration of the preadsorption and predesorption configurations and pressures for the smallest system.

Powered by Eventact EMS