
Photochemical Reduction of CO2 with Visible Light using a Polyoxometalate as Photoreductant
2Chemical Research Support, Weizmann Institute of Science, Rehovot
The reduction of CO2 to a higher energy species such as CO is a key transformation and by and large an important missing link towards the development of carbon-based solar fuels to remediate increasing amount of CO2 in the atmosphere and replace finite amounts of fossil fuels. Both photochemical and electrochemical pathways are being studied. The present state of the art teaches that the CO2 to CO reduction by
- a photochemical pathway requires sacrificial tertiary amines as the source of electrons and protons needed for the transformation and either low wavelength light or photosensitizers based on Ir and Ru compounds.1
- an electrochemical pathway that still requires prohibitively high potentials, typically higher than 1.7 V versus Ag/AgNO3.2
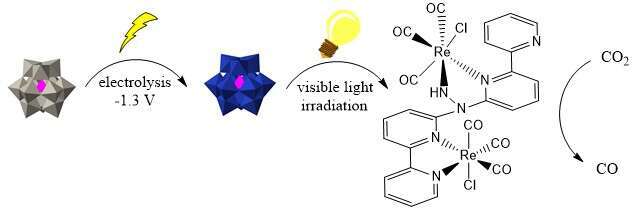
In order to overcome these two basic deficiencies, we combine a new di-rhenium molecular catalyst active for CO2 photoreduction that also has a tether to bind a polyoxometalate via a simple acid-base interaction. The polyoxometalate is an electron reservoir that can shuttle electrons from an electrode to the molecular catalyst.
Now, in a cascade of transformations a new photoelectrochemical pathway is presented wherein a polyoxometalate, the commercially available phosphotungstic acid, H3PW12O40, is electrochemically reduced at low potential (1.3 V versus Ag/AgNO3), and low intensity visible light (60 W tungsten lamp) is used to transfer electrons from the polyoxometalate to the catalyst that is active for selective reduction of CO2 to CO.
- a)Ziessel, R., Hawecker, J., Lehn, J.-M., Chim. Acta, 69, 1990–2012 (1986). (b) Fujita, E., Coord. Chem. Rev., 185-186, 373-384 (1999). (c) Takeda H., Koike K., Inoue H., Ishitani O., J. Am. Chem. Soc., 130, 2023-2031 (2008).
- Kumar, B., Llorente, M., Froehlich, J., Dang, T., Sathrum, A., Kubiak, C. P., Rev. Phys. Chem., 63, 541-569 (2102).

Powered by Eventact EMS