
Keynote Lecture
The Stability of Nitrogen-Centered Radicals
The stability of nitrogen-centered radicals can conveniently be defined relative to the aminyl radical (•NH2) as the unsubstituted (and thus unstabilized) parent system. These "radical stabilization energies" (RSEs) are identical to differences between N-H bond dissociation energies (BDEs) in ammonia (NH3) and the parent amines. Based on the same logic the stability of carbon and oxygen-centered radicals can be quantified and presented graphically as shown in Fig. 1. Using the reference systems H2O, NH3, and CH4 the RSE data for oxygen-, nitrogen-, and carbon-centered radicals can be combined in a global scale of BDE values (Figure 1). Using this type of presentation the reaction enthalpy of hydrogen transfer steps between amines and C- or O-centered radicals can be predicted in a straightforward manner. Using recently calculated data for a variety of nitrogen-centered radicals, substituent and environmental effects on N-radical stability will be discussed.
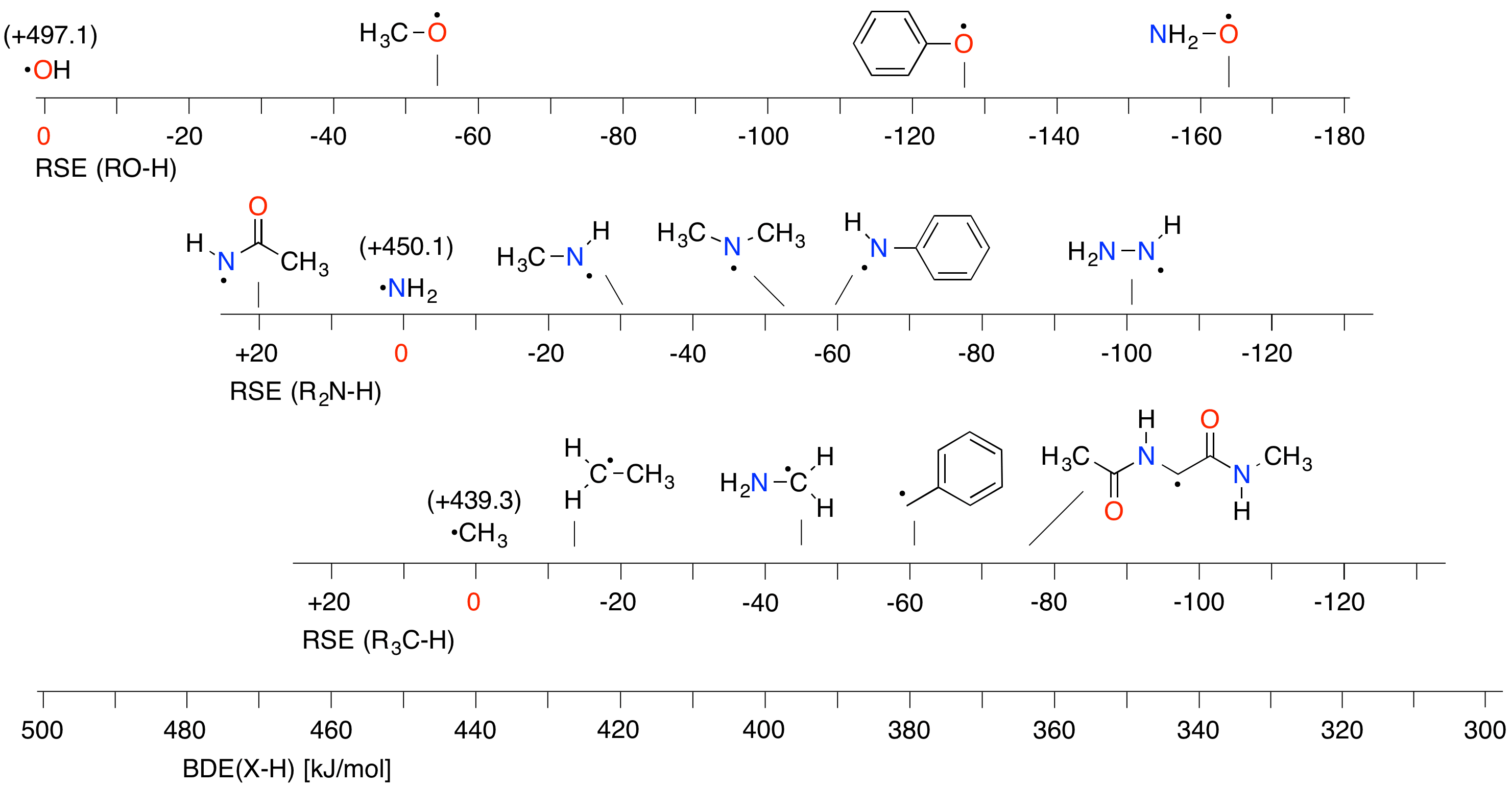
Figure 1. Radical stabilization energies (RSEs) for selected open shell systems (in kJ/mol).
References: (1) D. Sakic, H. Zipse, Adv. Synth. Catal. 2016, EarlyView. (2) J. Hioe, D. Sakic, V. Vrcek, H. Zipse, Org. Biomol. Chem. 2015, 13, 157. (3) J. Hioe, H. Zipse, RSC Advances 2013, 3, 12403.

Powered by Eventact EMS