
N-Aryl Imidazolines in Scaffold- and Lead-Oriented Synthesis
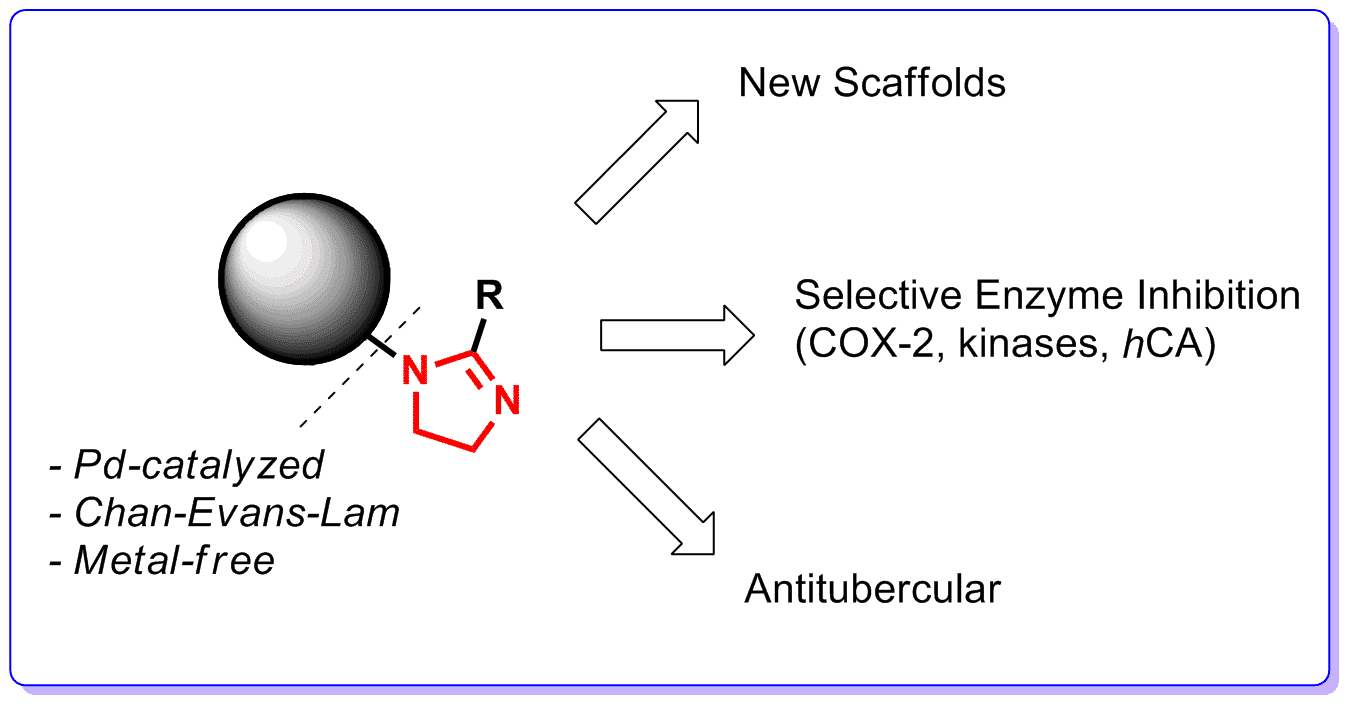
Our group has been active in the chemistry of 2-imidazolines where we particularly emphasized N-arylation approaches. In 2012, we reported a pinoneering method on Pd-catalyzed N-arylation of this special case of cyclic amidines and applied this approach toward the construction of COX-2, carbonic anhydrase and selective kinase inhbitory as well as antitubercular compounds. In 2016, we developed a room-temperature Chan-Evans-Lam procedure to introduce electron-neutral and electron-rich aromatic groups at the nitrogen atom of 2-imidazolines. This has now been applied to expand the SAR knowledge base around COX-2 and carbonic anhydrase inhibitors. Particularly intriguing is the prospect of achieve, in an intramolecular fashion, imidazoline N-arylation with no metal involved. We have identified sich an opportunity and recently applied it toward a novel approach toward medium-sized tings. The latter are of high interest as potential disruptors of therapeutically important protein-protein interactions.
References:
1. Krasavin, M. Novel diversely substituted 1-heteroaryl-2-imidazolines for fragment-based drug discovery. Tetrahedron Lett. 2012, 53, 2876-2880.
2. Mujumdar, P.; Grkovic, T.; Krasavin, M. A simple two-step access to diversely substituted imidazo[4,5-b]pyridines and benzimidazoles from readily available 2-imidazolines. Tetrahedron Lett. 2013, 54, 3336-3340.
3. Sarnpitak, P.; Mujumdar, P.; Morisseau, C.; Hwang, S. H.; Hammock, B.; Iurchenko, V.; Zozulya, S.; Gavalas, A.; Geronikaki, A.; Ivanenkov, Y.; Krasavin, M. Potent, orally available, selective COX-2 inhibitors based on 2-imidazoline core. Eur. J. Med. Chem. 2014, 84, 160-172.
4. Sarnpitak, P.; Mujumdar, P.; Taylor, P.; Cross, M.; Coster, M.J.; Gorse, A.-D.; Krasavin, M.; Hofmann, A. Panel docking of small-molecule libraries — Prospects to improve efficiency of lead compound discovery. Biotechnol. Adv. 2015, 33, 941-947.
5. Dar`in, D.; Krasavin, M. The Chan-Evans-Lam N-Arylation of 2-Imidazolines. J. Org. Chem. 2016 DOI: 10.1021/acs.joc.6b02404

Powered by Eventact EMS