
Strategy for the Development of Non-toxic Antimicrobial Cationic Amphiphiles
Fungal infections are an increasing problem both in Western medicine and in regions with limited healthcare availability. Mortality due to invasive fungal diseases likely exceeds that of tuberculosis or malaria with Candida albicans and Candida glabrata as the most frequently treated opportunistic fungal pathogens. Inspired by antimicrobial cationic peptides, we have developed several families of synthetic antimicrobial cationic amphiphiles. We demonstrated that by manipulating structural motifs it is possible to enhance their selectivity for microbial rather than mammalian red blood cell membranes. To date, none of the reported cationic amphiphiles exhibited membrane selectivity sufficient to be considered for development of membrane-disrupting antifungal agents.
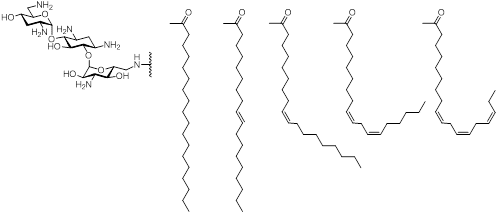
Here in we report that the incorporation of cis-double bonds into the lipids of cationic amphiphile significantly decrease their hemolysis and toxicity against mammalian cells. We demonstrated that increasing the degree of cis-unsaturation in the lipid of antifungal cationic amphiphiles does not affect their antifungal activity against Candida and decreases their hemolytic activity as well as their mammalian cell toxicity. One of the cationic amphiphiles with a linolenic acid lipid residue, containing three cis-double bonds, displayed no cytotoxicity against a panel of mammalian cell lines and primary cells. This compound selectively eradicated C. albicans cells while not affecting the viability of human cells in co-culture experiments. Our findings offer a new promising strategy for the development of non-toxic antimicrobial cationic amphiphiles safe for systemic antifungal treatment.

Powered by Eventact EMS