
Insight into the Thermal and Light-Activated Isomerization in Rhodopsin from QM/MM Simulations
The primary event of vision in the vertebrate eye is the highly selective and efficient photoisomerization of 11-cis-retinal protonated Schiff base bound to the visual protein rhodopsin (Rh). We investigated the possible consequences of the charge translocation associated with the photoisomerization.1 We show that the evolution of the electrostatic potential (ESP) projected by the chromophore onto the surrounding protein (Fig. 1) displays intense but topographically localized sudden variations in proximity of the decay region.2 pKa calculations carried out on selected snapshots and used as probes, indicate that the only residue which may be sensitive to the electrostatic potential shift is Glu181. Accordingly, our results suggest that the frail Tyr191/268-Glu181-Wat2-Ser186 hydrogen bond network may be perturbed by the transient variations of the electrostatic potential.
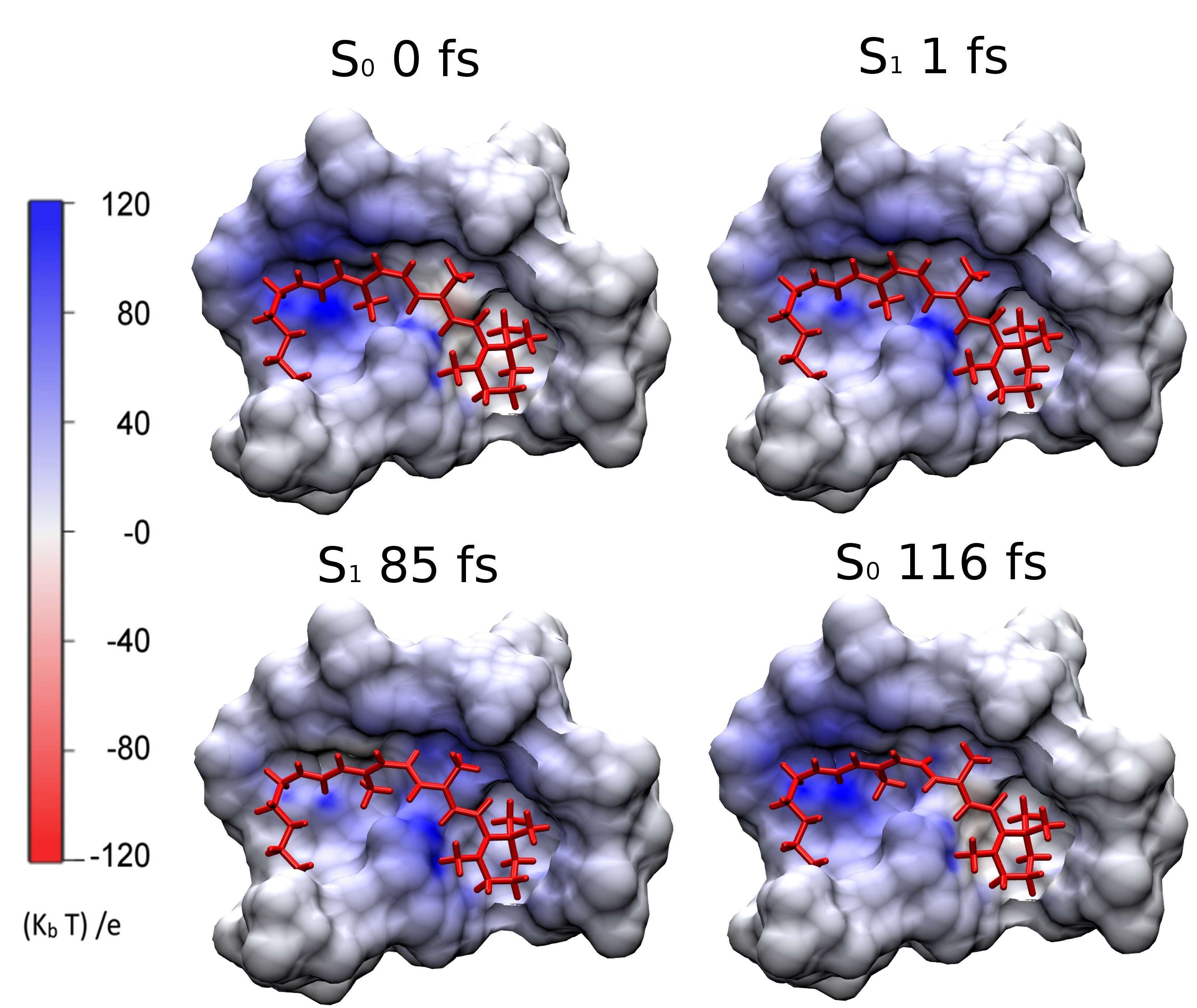
Figure 1 Trajectory snapshots documenting the ESP change on the chromophore cavity of bovine Rh.
[1] I. Schapiro, M. N. Ryazantsev, L. M. Frutos, N. Ferré, R. Lindh, M. Olivucci, J. Am. Chem. Soc. 133, 3354-3362, 2011
[2] F. Melaccio, N. Calimet, I. Schapiro, A. Valentini, M. Cecchini, M. Olivucci, J. Phys. Chem. Lett. 7, 2563 – 2567, 2016.

Powered by Eventact EMS