
Analyzing Amyloid Beta Aggregates with a Combinatorial Fluorescent Molecular Sensor
The self-assembly of amyloid beta (Aβ) peptides into insoluble aggregates is thought to play a major role in the progression of various neurodegenerative diseases, including Alzheimer`s disease (AD). Although various studies have shown that subtle variations in the dynamics and compositions of Aβ aggregates could have a significant impact on their physicochemical and pathological properties,1 currently there is no effective means to straightforwardly characterize the Aβ aggregation state. Fluorescent assays, which mainly rely on the ‘turn-on’ properties of a thioflavin T (ThT) molecule, can only detect the fibril formation, whereas other techniques that can determine the content of these assemblies require special expertise and are not high-throughput. To improve the ability to analyze Aβ aggregates, we have developed a combinatorial fluorescent molecular sensor that generate a wide range of unique emission ‘fingerprints’ upon binding to distinct Aβ aggregate species. The molecular sensor has been used to discriminate among aggregates generated from different alloforms (i.e., Aβ40 and Aβ42) or through distinct pathways, and it has also been used to track dynamic changes that occur in Aβ aggregation states, which result from the formation of low molecular weight (LMW) oligomers, high molecular weight (HMW), oligomers, protofibrils, and fibrils (Figure 1).
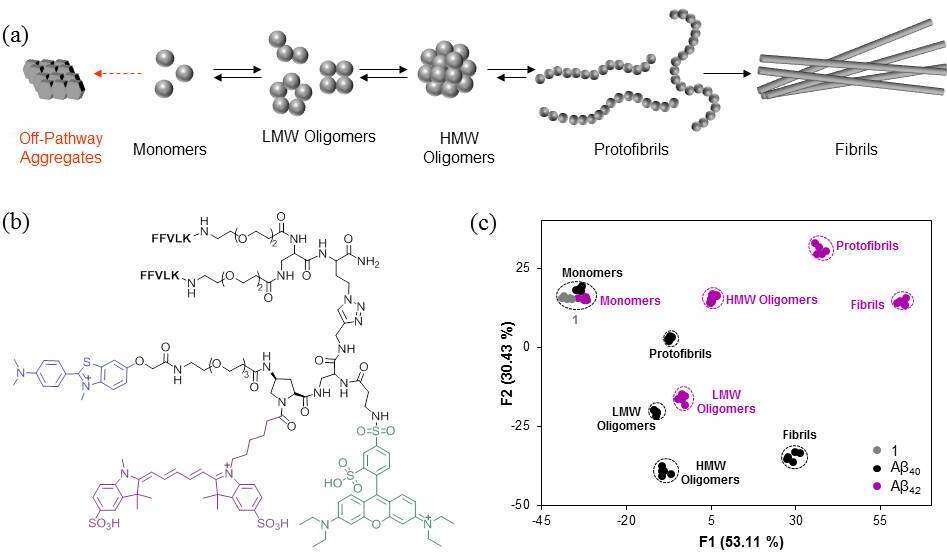
Figure 1. (a) Schematic representation of the Aβ aggregation process. (b) Chemical structure of a combinatorial fluorescent molecular sensor 1. (c) Linear discriminating analysis (LDA) showed the ability of sensor to discriminate among the various aggregates.
References.
1.Bitan, G.; Kirkitadze, M. D.; Lomakin, A.; Vollers, S. S.; Benedek, G. B.; Teplow, D. B., Proc. Natl. Acad. Sci. 2003, 100, 330; (b) Benilova, I.; Karran, E.; De Strooper, B., Nat. Neurosci. 2012, 15, 349.

Powered by Eventact EMS