
Keynote Lecture
Light-Induced Processes in Covalent Organic Frameworks
We explore the opportunities offered by spatially integrating photoactive molecular building blocks into a crystalline lattice based on the paradigm of covalent organic frameworks (COFs), thus creating models for organic bulk heterojunctions. In this presentation, we will address means of controlling the morphology and packing order of COFs through additives,(1) in thin films,(2) and with spatially locked-in building blocks.(3) Regarding the latter, the design of well-defined periodic docking sites enables us to achieve remarkably high crystallinity with several multidentate building blocks and a series of linear bridging units (see Figure 1).
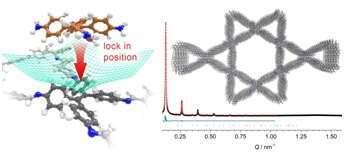
Figure 1. Docking site in COF network, representative diffractogram and stacked lattice of a docked dual-pore COF (3).
We will discuss different strategies aimed at creating electroactive networks capable of light-induced charge transfer. For example, we have developed a COF containing stacked thienothiophene-based building blocks acting as electron donors with a 3 nm open pore system, which showed light-induced charge transfer to an intercalated fullerene acceptor phase.(4) Contrasting this approach, we have designed a COF integrated heterojunction consisting of alternating columns of stacked donor and acceptor molecules, promoting the photo-induced generation of mobile charge carriers inside the COF network.(5) The great structural diversity and morphological precision that can be achieved with COFs make these materials excellent model systems for organic optoelectronic systems.
(1) M. Calik, T. Sick, M. Dogru, M. Döblinger, S. Datz, H. Budde, A. Hartschuh, F. Auras, T. Bein, J. Am. Chem. Soc. 2016, 138, 1234.
(2) D. D. Medina, J. M. Rotter, Y. H. Hu, M. Dogru, V. Werner, F. Auras, J. T. Markiewicz, P. Knochel, T. Bein, J. Am. Chem. Soc. 2015, 137, 1016.
(3) L. Ascherl, T. Sick, J. T. Margraf, S. H. Lapidus, M. Calik, C. Hettstedt, K. Karaghiosoff, M. Döblinger, T. Clark, K. W. Chapman, F. Auras, T. Bein, Nature Chem. 2016, 8, 310.
(4) M. Dogru, M. Handloser, F. Auras, T. Kunz, D. Medina, A. Hartschuh, P. Knochel, T. Bein, Angew. Chem. Int. Ed. 2013, 52, 2920.
(5) M. Calik, F. Auras, L. M. Salonen, K. Bader, I. Grill, M. Handloser, D. D. Medina, M. Dogru, F. Lobermann, D. Trauner, A. Hartschuh, T. Bein, J. Am. Chem. Soc. 2014, 136, 17802.

Powered by Eventact EMS