
Design and Synthesis of Highly Branched Organocatalysts
for Site–Selective Acylation
Site-selective acylation organocatalysts are a promising approach for preparing derivatives of polyhydroxylated natural products. Working toward this goal, we synthesized a molecular probe for acylation reaction that includes two reactive sites, which are situated in different environment (polar and a-polar). Such design allows examining the tendency of various catalysts to perform acylation at a specific site (Scheme 1).
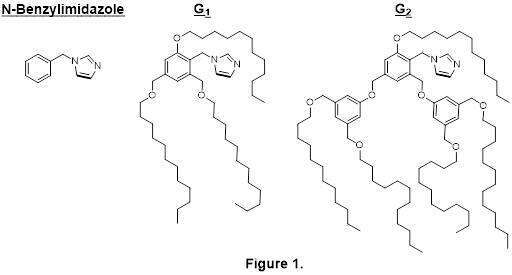
We envisaged using the dendritic architecture in order to create a catalytic pocket of a specified polarity. Thus, we conceived a catalytic system composed of a dendrimer that incorporates a polar imidazole active site in the interior and an a-polar periphery that will create a hydrophobic envelopment of this catalytic site. We presume that the polarity differences between the dendrimer regions will impact the probe access path into the catalytic pocket and, consequently, allow a preferred reaction at a particular site.
The designed catalysts (Figure 1) were synthesized, examined under several standard sets of conditions and compared to a simple non-dendritic analogue. The catalytic experiments revealed several trends, reflecting the effects of the probe concentration, solvent polarity and the nature of the acylation reagent. Based on these findings, preference for the desired site of the proe can be achieved by providing specific conditions and a specific catalyst.

Powered by Eventact EMS