DIIRON MACROCYCLIC CATALYSTS FOR OXIDATION, OXIDATIVE DEHALOGENATION AND C-C BOND FORMATION
IRCELYON, CNRS - Université Lyon 1
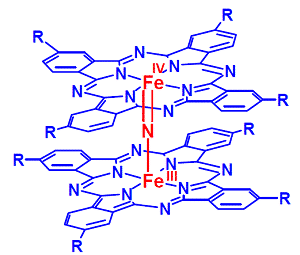
N-Bridged diiron phthalocyanine and porphyrin complexes show rich redox chemistry and exist in several stable high oxidation states: LFeIII(µN)FeIVL (resting state, L= phthalocyanine or porphyrin), LFeIV(µN)FeIVL (1e- oxidation) and LFeIV(µN)FeIV(L∙+) (2e- oxidation).1 Given corresponding high valent diiron species with oxo axial ligand can be prepared they should be very powerful oxidants. We have found that LFeIII(µN)FeIVL complexes are able to activate peroxides (H2O2, tBuOOH and m-CPBA) to form different high-valent diiron oxo species which were obtained at low temperatures and characterized.2 A strong beneficial effect of the binuclear macrocyclic platform on the oxidation properties was shown in the oxygen transfer to alkanes with strong C-H bonds and olefins by kinetic comparison with mononuclear counterparts. These novel catalysts were efficient in several oxidation,3,4 oxidative dehalogenation1 and C-C bond forming reactions5 providing high turnover numbers and high product yields. Mechanistic background of the versatility and efficiency of macrocyclic diiron complexes in difficult-to-perform reactions will be discussed.
 References
References
1. P. Afanasiev, D. Bouchu, E.V. Kudrik, J.M.M. Millet, A.B. Sorokin, Dalton Trans. 2009, 9828-9836.

2. P. Afanasiev, E.V. Kudrik, J.M.M. Millet, D. Bouchu, A.B. Sorokin, Dalton Trans. 2011, 40, 701-710.
3. A.B. Sorokin, E.V. Kudrik, D. Bouchu, Chem. Commun. 2008, 2562-2564; A.B. Sorokin, E.V. Kudrik, L.X. Alvarez, P. Afanasiev, J.M.M. Millet, D. Bouchu, Catal Today 2010, 157, 149-154; Ü. İşci, P. Afanasiev, J.M.M. Millet, E.V. Kudrik, V. Ahsen, A.B. Sorokin, Dalton Trans. 2009, 7410-7420.
4. E.V. Kudrik, A.B. Sorokin, Chem. Eur. J. 2008, 14, 7123-7126.
5. L.X. Alvarez, E.V. Kudrik, A.B. Sorokin, Chem. Eur. J. 2011, 17, 9298-9301.
Powered by Eventact EMS