
Mechanism of the Copper/TEMPO Catalyzed Aerobic Oxidation of Alcohols
2Department of Chemical Research Support, Weizmann Institute of Sciences, Rehovot
Identifying the mechanism of a catalytic reaction is paramount for designing new and improved catalysts. Several alternative catalytic cycles for the copper-TEMPO catalyzed aerobic oxidation of alcohols to the corresponding aldehydes or ketones were examined in their entirety using density functional theory at the SMD(CH3CN)-RIJCOSX-DSD-PBEB95/def2-TZVP//DF-PBED3BJ/def2-SVP level of theory.[1] A novel catalytic cycle in which TEMPO remains coordinated to copper throughout, was identified as the most likely mechanism. There are three components to the catalytic cycle: (1) hydrogen transfer from the alkoxy ligand to coordinated TEMPO (2) oxygen activation with formation of a peroxo complex, and (3) alcohol activation with transfer of the O–H proton to the peroxo ligand. The oxidation takes place via a six-membered intramolecular hydrogen transfer transition state. Importantly, this is not the rate determining step. Instead, the rate determining step involves oxygen activation and/or the initial alcohol activation.
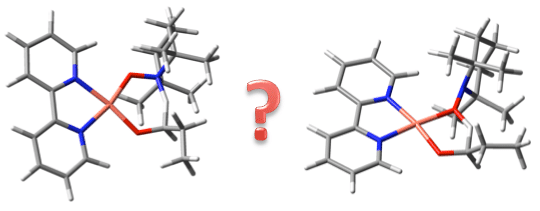
[1] Mechanism of the Copper/TEMPO Catalyzed Aerobic Oxidation of Alcohols Mark A. Iron and Alex M. Szpilman* Chemistry a European Journal, 2016, In Press (HOT paper)

Powered by Eventact EMS