AEROBIC OXIDATION OF PERFLUOROSUBSTITUTED ALCOHOLS CATALYZED BY Pt(II)phenanthroline COMPLEXES WITH ANCILLARY CATIONIC MOIETIES
Fluorine containing compounds are of increasing interest and importance in various fields related to agricultural and pharmaceutical chemistry. For example, it is known that fluorinated ketones can inhibit the function of various enzymes, in particular, many hydrolytic enzymes are very efficiently inhibited by trifluoromethyl ketones. One of the better ways of incorporating fluorine into molecules is to convert fluorine containing building blocks to reactive target compounds – e.g. fluoro-aldehydes and fluoro-carboxylic acids. The simple structured, highly reactive aldehyde group of trifluoroacetaldehyde is one of the highly applicable fluorine-contained building blocks, where, in addition, its pro-chiral property is utilized in many CF3-containing chiral compounds.
The oxidation of alcohols, such as ethanol to the corresponding acetaldehyde or acetic acid typically catalyzed by palladium complexes, proceeds via initial C-H bond activation. In the case of fluoroalcohols, for example trifluorethanol, this C-H activation is generally completely inhibited due to the strong electron withdrawing effect of the adjacent fluorine atoms. In our research we have observed by serendipity that oxidations of fluoroalcohols are possible using platinum(II)phenanthroline complexes with ancillary cationic moieties, Scheme.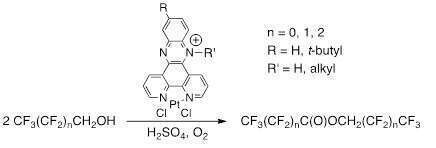
Powered by Eventact EMS