PHOTO INDUCED OXIDATION OF Fe(N4Py) AND RELATED COMPLEXES
Stratingh Institute for Chemistry, University of Groningen, Groningen
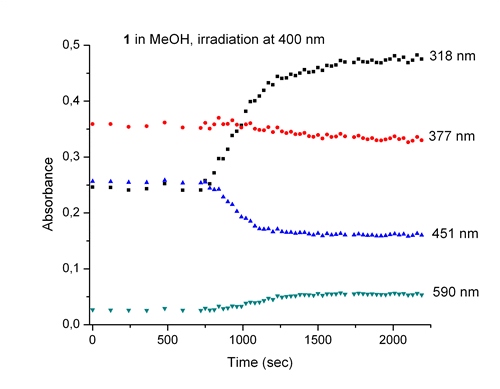
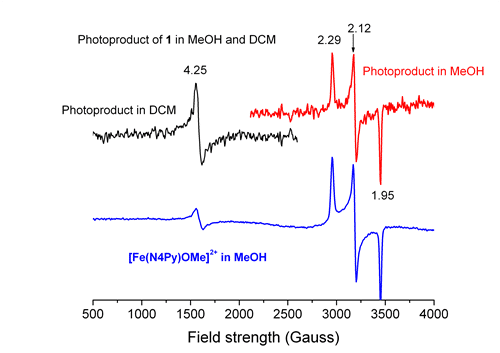
The complex [Fe(N4Py)(CH3CN)](ClO4)21 (1) is one of the most active Fe based oxidation-catalysts reported and has been used in DNA cleavage2 and oxidation catalysis.3 The activity towards DNA cleavage by 1 was found to be enhanced under UV/Vis irradiation.4 This activity increase is dependent on the structure of the complex and the wavelength and power of irradiation. In order to understand the origin of the photo-enhanced DNA cleavage, a study of the photochemistry of this complex itself is necessary. Here we report on the photochemistry of 1 and its alkylated analogue [Fe(MeN4Py)(CH3CN)](ClO4)2 (2).5 Irradiation of aqueous, dichloromethane or methanolic solutions of 1 and 2 leads to formation of FeIII species. Addition of ascorbic acid to the photo product recovers the UV/Vis spectrum of 1 and 2, fully indicating electron transfer oxidation. Oxygen was found to be the terminal oxidant. Recently we demonstrated that in the aqueous solutions of complexes 1 and 2, there are equilibria between two different species and spin states.5 The equilibrium between spin states in solution is proposed to be the origin of the effectiveness of 1 in cleaving DNA in water with 3O2 as terminal oxidant and of the photochemical enhancement of this process.
References
(1) M. Lubben, A. Meetsma, E. C. Wilkinson, B. L. Feringa, L. Que, Jr., Angew. Chem. Int. Ed. Engl. 34(1995) 1512.
(2) G. Roelfes, M. E. Branum, L. Wang, L. Que, Jr., B.L. Feringa, J. Am. Chem. Soc.122(2000) 11517.
(3) G. Roelfes, M. Lubben, R. Hage, L. Que, Jr., B. L. Feringa, Chem. Eur. J. 6(2000) 2152.
(3) G. Roelfes, M. Lubben, R. Hage, L. Que, Jr., B. L. Feringa, Chem. Eur. J. 6(2000) 2152.
(4) Q. Li, W. R. Browne, G. Roelfes, Inorg.Chem. 49(2010) 11009.
(5) A. Draksharapu, Q. Li, H. Logtenberg, T. A. van den Berg, A. Meetsma, J. Scott Killeen, B. L. Feringa, R. Hage, G. Roelfes, W. R. Browne, Inorg. Chem. 51(2012) 900.
Powered by Eventact EMS