PROTONATION OF POLYOXOMETALATES AND ITS IMPLICATIONS FOR MARS-VAN KREVELEN MECHANISM
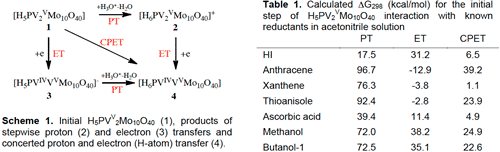
Redox reactions of polyoxometalates (POM) usually follow the Mars-van-Krevelen (MvK) mechanism, in which electron transfer may or may not precede oxygen transfer. For several reactions, the outer-sphere reduction of POMs has been proven to precede oxygen transfer. In this case, the electron transfer (ET) is typically accompanied by proton transfer (PT) that can precede, follow or be concerted (CPET) with the ET, Scheme 1. Some other studies indicate that chemisorption and inner-sphere activation of a reductant presents a key stage of the interaction. In this case the working form of the catalysts involves partial decomposition of the POM with formation of coordinatively unsaturated metal sites (CUS). Rational choice of the possible reaction mechanisms requires accurate energetic data on the redox reactions shown in Scheme 1 and on the stability and catalytic behavior of the CUSs. For POMs these properties strongly depend on protonation. Recently, we applied high-level DFT calculations to study protonated Keggin structures H3+xPVxMo12‑xO40 (x= 0, 1, 2).[1] In the present work we extended our study of phosphovanadomolybdates H5PV2Mo10O40 (1) to calculations of their protonated (2) and reduced (3,4) forms (Scheme 1). We considered untouched Keggin structure as well as hypothetical defect structures in which one, two or three metal-oxygen bonds around the same metal atom are broken, and their interactions with HX reductants. This allows us to get the thermodynamics of the sequential and concerted outer-sphere proton–electron transfers between H5PV2Mo10O40 and several known electron donors (Table 1) followed by the reactant coordination and activation on hitherto unreported metal surface defects. Total thermodynamic effect of H5PV2Mo10O40 interaction with the selected substrates sheds additional light on the mechanisms of their interactions and allows to explain several experimental observations.
[1] I. Efremenko, R. Neumann. J. Phys. Chem. A 2011, 115, 4811–4826.
Powered by Eventact EMS