COPPER-FREE PALLADIUM CATALYZED SELECTIVE ANTI-MARKOVNIKOV OXIDATION OF ALKENES
1
1
2
1
1
1Stratingh Institute for Chemistry, University of Groningen, Groningen
2Innovative Synthesis, DSM, Geleen
2Innovative Synthesis, DSM, Geleen
The selective catalyzed oxidation of alkenes is of considerable interest in the generation of value added materials from bio-available feedstock. In PdII-catalysed oxidation of alkenes to carbonyl compounds, usually referred as the Wacker reaction[1], terminal alkenes typically yield the corresponding ketones. However, the aldehyde products are obtained occasionally for specific substrates [2]. In this presentation we report our recent work on the development of methods for the anti-Markovnikov oxidation of alkenes to aldehydes in environmentally friendly solvents. Furthermore, the use of stoichiometric copper salts is avoided, allowing for cleaner reactions. (scheme 1) Styrene aldehyde was obtained as the major product from styrene under these reaction conditions (scheme 2). Reaction progress monitoring by UV.Vis and Raman spectroscopy as well as kinetic analyses will be described also(figure 1).
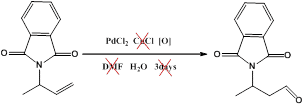
Scheme 1

Scheme 2
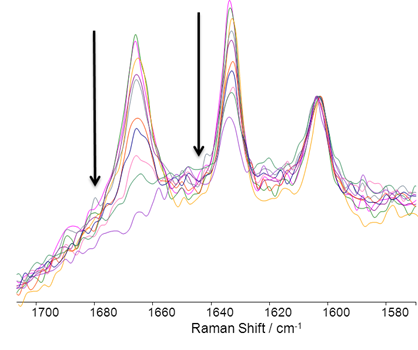
Figure 1
[1] J. Tsuji, H. Nagashima, and H. Nemoto Org. Synth., 1990, 7, 137
[2] (a) B. Weiner, A. Baeza, T. Jerphagnon, and B. L. Feringa J. Am. Chem. Soc. 2009, 131, 9473
Powered by Eventact EMS