OBSERVATION OF Fe(V)=O USING VARIABLE-TEMPERATURE MASS SPECTROMETRY AND ITS ENZYME-LIKE C-H AND C=C OXIDATION REACTIONS
2WestCHEM, School of Chemistry, University of Glasgow, Glasgow
3Institut de Química Computacional, Universitat de Girona, Girona
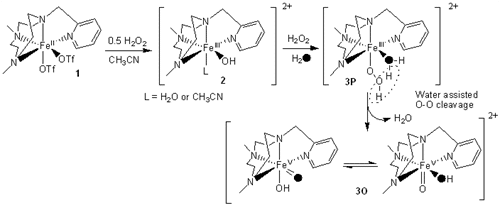
Oxo transfer chemistry mediated by iron underpins many biological processes and today is emerging as synthetically very important for the catalytic oxidation of conventionally hard to activate C-H and C=C moieties1-4. Despite the vast amount of research in this area, experimental characterization of the reactive species under catalytic conditions is very limited, although a Fe(V)=O moiety has been postulated5,6. Herein, using temperature variable mass spectrometry (TV-MS), we show the generation of a Fe(V)=O species, [FeV(O)(OH)(Me,HPytacn)]+ 3O, at -40ºC and its reaction with an olefin, using the synthetic non-haem complex [FeII(Me,HPytacn)(OTf)2] 1 (Me,HPytacn = 1-(2’-pyridylmethyl)-4,7-dimethyl-1,4,7-triazacyclononane). Also, with isotopic labelling experiments we were able both to follow oxygen-atom transfer from H2O2 / H2O through Fe(V)=O to the products and to probe the reactivity as a function of temperature. This is the first time such highly reactive intermediate has been observed and the first ever implementation of temperature variable mass spectrometry to investigate reactive intermediates7.
References
- Que, L.; Tolman, W. B. Nature, 2008, 455, 333.
- Costas, M.; Mehn, M. P.; Jensen, M. P.; Que, L. Jr. Chem. Rev., 2004, 104, 939.
- Chen, M. S.; White, M. C. Science, 2007, 318, 783.
- Chen, M. S.; White, M. C. Science, 2010, 327, 566.
- Company, A.; Gómez, L.; Güell, M.; Ribas, X.; Luis, J. M.; Que, Jr. L.; Costas, M. J. Am. Chem. Soc. 2007, 129, 15766.
- Company, A.; Feng, Y.; Güell, M.; Ribas, X.; Luis, J. M.; Que, Jr. L.; Costas, M. Chem. Eur. J. 2009, 15, 3359.
- Prat, I.; Mathieson, J. S.; Güell, M.; Ribas, X.; Luis, J. M.; Cronin, L.; Costas, M.; Nature Chemistry 2011, 3, 788.
Acknowledgements: This work was funded by MICINN for project CTQ2009-08464 and the European Research Foundation for Project ERC-2009-StG-239910
Powered by Eventact EMS