ELECTRONIC EFFECTS ON SINGLE SITE IRON CATALYST WATER OXIDATION
Departament de Química, Universitat de Girona, Girona
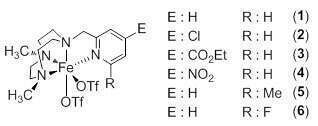
Schematic representation of the catalysts used in this work
1. Brimblecombe, R., Swiegers, G. F., Dismukes, G. C. & Spiccia, L.Angew. Chem. Int. Ed., 2008, 47, 7335.
2. Ellis,WC., McDaniel, ND., Bernhard,S., Collins, T.J., J. Am. Chem. Soc., 2010, 132, 10990.
3. Kanan, M. W. & Nocera, D. G, Science,,2008, 321, 1072.
4. Qiushi Yin, J. M. T., Claire Besson, Yurii V. Geletii, Djamaladdin G. Musaev, Aleksey E. Kuznetsov, Zhen Luo, Ken I. Hardcastle, Craig L. Hill Science, 2010, 328, 34.
5. Lloret Fillol, J., Codolà, Z., Garcia-Bosch, I., Gómez, L., Pla, J.J., Costas, M. Nat.Chem, 2011, 3, 807.
Powered by Eventact EMS