CASCADE EPOXIDATION OF ALKENES BY "IN SITU" FORMED CUMYL HYDROPEROXIDE OVER BIFUNCTIONAL COPPER CATALYST
Epoxides are valuable and versatile synthetic intermediates used in the production of epoxide resins, plasticizers, cosmetic and others, but actually their production routes overlook energy, atom efficiency and environmental concerns. Since the importance of these chemicals, more affordable production routes are ever pursued [1].
Hydroperoxides are widely used for the industrial production of epoxides in a two steps process: first the hydroperoxide is produced by reaction with O2, then the epoxide is formed from the olefin and the oxidizing compound [2]. In this view, cascade processes in which the hydroperoxide, generated in situ, is immediately available for the epoxidation reaction, without problems of storage and transportation, are very attractive [1].
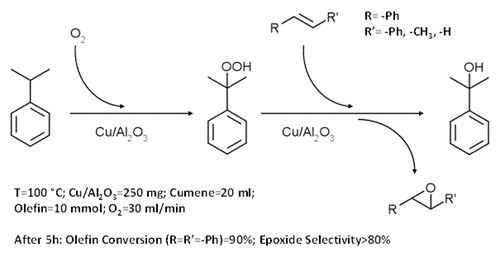
Here we wish to report our result in the one-pot epoxidation of alkenes mediated by the in-situ formation of cumyl hydroperoxide (CHP) on supported copper catalysts.
In our work, the reaction was performed at 100 °C in a flask, operating at atmospheric pressure of molecular oxygen (bubbling), in the presence of stilbene, cumene and Cu/Al2O3 catalyst, without iniziators or promoters. The results indicate the excellent reactant conversion and epoxide selectivity of the entire process, that occurs via catalytic oxidation of cumene to CHP followed by the catalytic epoxidation of stilbene with CHP. Indeed the separate study of the two reactions highlighted the fundamental role of the copper catalyst on both steps.
The work indicated also the possibility to extend this process to other olefins, such as styrene.
Catalyst recyclability tests, metal leaching analyses, as well as morphological characterization of the catalyst (HR-TEM, TPR,…) are still in progress.
References
[1] C. Aprile, A. Corma, M. E. Domine, H. Garcia, C. Mitchell, J. Catal. 264 (2009) 44.
[2] F. Cavani, J. H. Teles, ChemSusChem 2 (2009) 508.
Powered by Eventact EMS