DIVERTING SELECTIVITY IN THE OXIDATION OF NON-ACTIVATED ALKYL C-H BONDS WITH H2O2 USING A FAMILY OF STERICALLY HINDERED IRON CATALYSTS
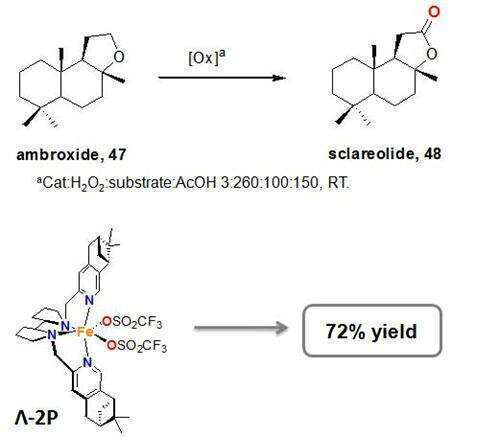
The catalytic transformation of sp3 C-H bonds into more reactive C-OH or C=O remains a challenging task in chemical synthesis. In current methods, selectivity and conversion of the substrate into products are still limited.1 In a recent paper we showed a marked increase in efficiency and robustness in the oxidation of C-H bonds with H2O2 using iron catalysts where bulky alkyl groups were fused to the pyridine rings of tetradentate N-based ligands. This improvement was more important when the ligand formed a well defined cavity around the metal center.2 Further exploration of this strategy has now been pursued in the bpbp ligand, giving rise to novel chiral complexes (Λ-2P and ∆-2P) that proved efficient catalysts for the oxidation of alkyl C-H bonds.
Herein we present an study on the catalytic activity of the full family of catalysts, and we show remarkable behavior in the oxidation of complex organic molecules such as natural products. Good product yields are attained using H2O2 as oxidant, and low catalyst loadings, without the need of excess of substrate. This family of catalysts also shows improved regioselectivities with regard to that attained with conventional C-H oxidizing agents, and less structured iron catalysts. 3 Most interestingly, we prove that regioselectivity can be directed by choosing the appropriate combination of catalyst and protecting group of the substrate or reaction conditions. Structural elements of the catalysts contributing to C-H site selectivity have been identified and include an unprecedented chiral match between catalyst and substrate.
1. Chen, M. S., White, M. C. Science. 2007, 318, 783-787.
2. Gómez, L., Garcia-Bosch, I., Company, A., Benet-Buchholz, J., Polo, A., Sala, X., Ribas, X., Costas, M. Angew. Chem., Int. Ed. 2009, 48, 5720-5723.Newhouse, T.; Baran, P. S.,
Acknowledgements: Generalitat de Catalunya (FI-DGR 2011, ICREA Academia and SGR 2009-SGR637), MICINN (CTQ 2009-08864 and Consolider IngenioCSD2010-00065), ERC-StG-239910.
Powered by Eventact EMS