MECHANISTIC INSIGHT OF HOMOGENEOUS WATER OXIDATION CATALYSIS WITH IRON COORDINATION COMPLEXES
Water oxidation WO catalysis constitutes the bottleneck for the development of energy conversion schemes based on sunlight. State of the art homogeneous WO catalysis is so far efficiently performed mostly with earth-scarce transition metals while 3d metal-based complexes are much less established.[1-3] We show that readily available, environmentally benign iron coordination complexes catalyze homogeneous water oxidation to O2, with high efficiency during hours. Turnover numbers > 350 and >1000 were obtained when using cerium ammonium nitrate (CAN) at pH 1, and NaIO4 at pH 2, respectively.[4]
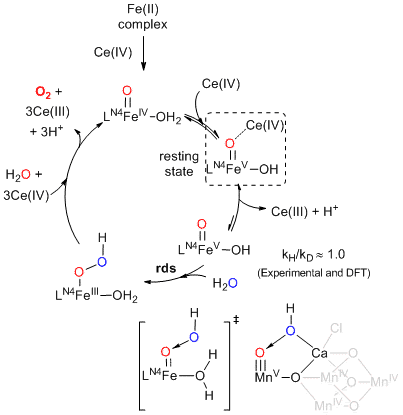
This communication will be focus on the mechanistic aspects. In particular, kinetic studies, spectrometric, spectroscopic monitoring, isotopic effects, isotopic labeling, electronic effects and DFT computational calculations have been used to get insights into the mechanism of the catalytic reactions. The role of the high oxidation state oxo-iron (IV) and (V) as resting state and as responsible for the O-O forming event. Finally, a mechanism will be discussed.
Powered by Eventact EMS