IRON CATALYZED C-H HYDROXYLATION AND OLEFIN Cis-DIHYDROXYLATION WITH A SINGLE ELECTRON OXIDANT AND WATER AS OXYGEN ATOM SOURCE. A MODEL FOR O2-INDEPENDENT OXIDATIVE ENZYMES
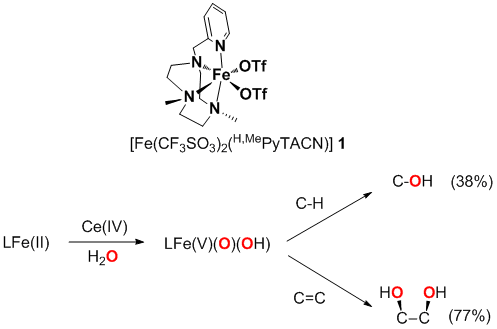
Rieske Dioxygenases are a family of enzymes that catalyze the cis-dihydroxylation of arenes and the hydroxylation of alkyl C-H bonds in alkyl aromatics using O2 as oxidant. It has been suggested that a they operate through a common mechanistic scenario with the better established cytochrome P-450, involving a FeV(O)(OH) species as responsible for substrate attack.[1] Non-heme mononuclear iron complex [Fe(CF3SO3)2(Me,HPyTACN)] 1 is a functional model of this family of enzymes and has proven particularly effective as catalysts for the efficient hydroxylation of alkanes and the oxidation of alkenes, employing H2O2 as oxidant. [2] We have also recently described that complex 1 reacts with cerium ammonium nitrate (CAN) in aqueous solutions eliciting efficient water oxidation.[3] Isotopic, product analyses and computational methods have lead to the conclusion that a highly reactive FeV(O)(OH) species is finally responsible for stereospecific hydroxylation of alkyl C-H bonds, epoxidation and cis-dihydroxylation of olefins, resembling the oxygenase P-450 like pathway proposed for Rieske Dioxygenases.
Herein we show that oxidizing species formed in reactions of complex 1 with CAN are indeed competent intermediates for eliciting stereospecific C-H hydroxylation, and olefin cis-dihydroxylation, and that water is the source of oxygen atoms in these reactions. These reactions are remarkable because they are fundamentally different from canonical oxygenase-like reactions where high valent iron-oxo species are formed by dioxygen activation or reaction with oxo-donors. Instead, reactions described herein may serve as a functional model for O2-independent oxidative enzymes.
[1] A. Karlsson, et al., Science 2003, 299, 1039.
[2] (a) A. Company et al., J. Am. Chem. Soc. 2007, 129, 15766. (b) I. Prat, et al. Nat. Chem. 2011, 3, 788.
[3] J. Lloret, et al. Nat. Chem. 2011, 3, 807.
Powered by Eventact EMS