GREENER OXIDATION OF PRIMARY, SECONDARY ALCOHOLS AND DIOLS UNSING Fe(III) COMPLEX
Selective oxidation of alcohols to the corresponding carbonyl compounds is a fundamental transformation in organic synthesis.1In particular, selective oxidation of alcohols to the carbonyl compounds such as primary alcohols to aldehydes or acids, or oxidation of secondary alcohols to ketones is attractive because the target molecules can be obtained directly in one-pot sequences. Many catalytic methods have been developed for oxidation of aliphatic alcohols. despite of these improvements in the catalyst development, addressing these challenges will require more efficient, cheaper, chemoselective, and greener catalyst; the low cost and non-toxicity of iron render it a particularly attractive metal in this regard.
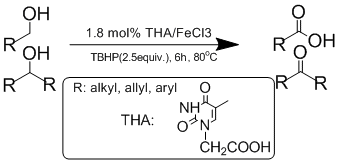
Scheme 1.Thymine acetate iron complex catalyzed oxidation of alcohols to acids and ketones.
References
1. Tojo G., and Fernandez, M.,Oxidation of primary alcohols to carboxylic acids, Springer, Berlin, 2006; Tojo, G. and Fernandez, M.Oxidation of Alcohols to Aldehydes and Ketones, Springer, Berlin, 2006; Backwall, J. E.Modern Oxidation Method,wiley-VCH, New York, 2004; M. Hudlicky,Oxidation in Organic Chemistry,Am. Chem. Soc,Washington, DC, 1990;. Sheldon R. AIn dioxygen activation and Homogeneous Catalytic Oxidation(Ed.: L. L. Simandi), Elsevier, Amestrdam, 1991, P573.
2- Al-Hunaiti A.,Niemi T., Siboueh A., Pehko P., Leskela M., Repo T. J. Chem. Comm. 2010, 46, 9250-9252.
Powered by Eventact EMS