
ICS Young Scientist Prize
Chiral Iron Phosphate Complexes for Asymmetric Cross-Dehydrogenative Coupling Reactions
Iron-catalyzed oxidative cross-coupling reactions provide an environmentally friendly strategy to form new C-C bonds directly from two C-H bonds. The biomimetic oxidative cross-coupling of phenolic components suffers from a lack of selectivity, making the cross-coupling of two non-identical reactants at specific sites difficult to obtain. To address the selectivity issue in these reactions, my group developed a set of highly selective oxidative cross-coupling reactions catalyzed by different iron catalysts that follow distinct coupling mechanisms. We applied kinetic studies, electrochemical methods and DFT calculations to elucidate some of the factors that control the chemoselectivity and regioselectivity in phenol-phenol and phenol-arene cross-coupling reactions. We then developed a prediction model to anticipate the feasibility of pairs of phenols to favor cross-coupling over homocoupling, thereby enabling efficient synthesis of specific phenolic architectures with high degrees of chemoselectivity and regioselectivity.
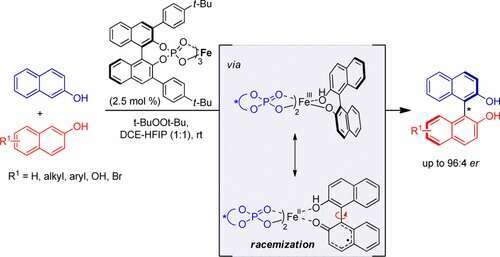
The presentation will focus mainly on our efforts to address the asymmetry challenge in cross-dehydrogentaive coupling (CDC) reactions. For that purpose, novel chiral iron phosphate complexes were prepared as catalysts for the synthesis of enantio-enriched C1- and C2-symmetric 1,1`-bi-2-naphthols (BINOLs) and for the stereoselective CDC between 2-naphthols and a-subsituted-b-ketoesters. A destructive BINOL racemization process that competes with the enantioselective oxidative coupling of 2-naphthols was revealed, thereby offering new insights into this highly important reaction.
Selected references
Narute, S.; Parnes, R.; Toste, F. D.; Pappo, D. J. Am. Chem. Soc. 2016, 138 (50), 16553–16560.
Dyadyuk, A.; Sudheendran, K.; Vainer, Y.; Vershinin, V.; Shames, A. I.; Pappo, D. Org. Lett. 2016, 18 (17), 4324–4327.
Libman, A.; Shalit, H.; Vainer, Y.; Narute, S.; Kozuch, S.; Pappo, D. J. Am. Chem. Soc. 2015, 137, 1453-11460.
Gaster, E.; Vainer, Y.; Regev, A.; Narute, S.; Sudheendran, K.; Werbeloff, A.; Shalit, H.; Pappo, D. Angew. Chem. Int. Ed. 2015, 54 (14), 4198-4202.

Powered by Eventact EMS