Use of electrochemical impedance spectroscopy (e.i.s) for the analysis of diffusion and semi conductive properties for passive α-PbO growth on Pb and Pb-Sn alloys
2Institut Jean Lamour, Université de Lorraine
Passivation of lead and lead-tin alloys under deep discharge conditions of the lead acid batteries is investigated at an anodic potential of +0.7 Volts Vs Hg/Hg2SO4/K2SO4 sat in 0.5M H2SO4 solution using e.i.s
In these conditions p-type semi conductive lead monoxide (α-PbO) is formed and provokes a premature capacity loss due to the electrical passivation of the metal, acting as an electrical barrier at the grid surface, hindering the recharge of the battery.
The experimental impedance data have been simulated by an electrical equivalent circuit that includes:
- the space charge capacitance Csc describing the semi conductive properties of PbO and represented by a constant phase element (CPE) in order to take into account the dispersion of the capacitance value.
-the diffusion impedance ZD where:
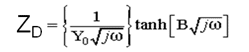
Related to transport of anions O-2 in a finite region having a thickness l and modulated by two parameters (Y0 and B) indicating that the PbO growth is due to a solid state diffusion under local electric field.
From the analysis of the impedance results the diffusion coefficient D is determined and found to reach 6.6 10-9 cm2 /Sec.
We have found a value close to that (7.0 10-9 cm2 /Sec) for an anodized Mg alloy after 20h immersion in the corroding ASTM water and related to the mass transport of Mg+2 in the pores of the anodic oxide layer.
The effect of low and high concentration of Sn added on the thickness of PbO is discussed in terms of the internal electric field and the composition of the oxide.
Mott-Schottky plots have been drawn, the concentration of the charge carriers NA determined and the semi conductive and corrosion properties of the oxides interpreted.

Powered by Eventact EMS