
Nitrogen Lewis Acids
Lewis acids and their interactions with Lewis bases are widely used in many aspects of chemistry. Although many chemical elements can serve as central atom of Lewis acids, nitrogen-centered compounds are usually considered as Lewis bases due to their lone pair of electrons having relatively high energy.
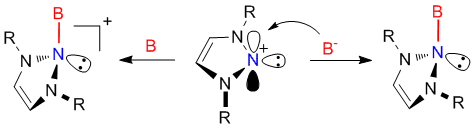
Recently, our group demonstrated that N-heterocyclic nitrenium ions can coordinate to various transition metal centers[i], possessing a Lewis basic feature. The availability of vacant pp-orbital of nitrenium species suggests that it also might be involved in s-interactions with Lewis bases, possessing a Lewis acidic character.
Here we present the first examples of reactivity of nitrenium species towards Lewis bases. This represents a first example of robust, stable and stereoelectronically modifiable nitrogen Lewis acids, which form well-defined adducts with Lewis bases.[ii] Moreover, this reactivity provides a new type of stable organic compounds, called triazanes, bearing all-saturated three consecutive nitrogen atoms.
[i] (a) Tulchinsky, Y.; Iron, M. A.; Botoshansky, M.; Gandelman, M. Nature Chem. 2011, 3, 525. (b) Tulchinsky, Y.; Kozuch, S.; Saha, P.; Botoshansky, M.; Shimon, L.; Gandelman, M. Chem. Sci. 2014, 5, 1305. (c) Tulchinsky, Y., Kozuch, S., Saha, P., Mauda, A., Nisnevich, G., Botoshansky, M., Shimon, L., Gandelman, M. Chem. Eur. J. 2015, 21, 1-13.
[ii] Pogoreltsev, A., Tulchinsky, Y., Fridman, N., Botoshansky, M., Gandelman, M. JACS, in press.

Powered by Eventact EMS