
Nitrenium-Based Iridium Complexes
N-heterocyclic carbenes are well known and commonly used as ligands in organometallic chemistry. However, their analogue ligands based on isoelectronic nitrogen cation atom has only recently been accomplished by our group. A new type of cationic nitrenium-based PNP pincer ligands, bearing two phosphine based arms, successfully coordinates to neutral, cationic and dicationic late transition metal centers (Scheme 1).[1]
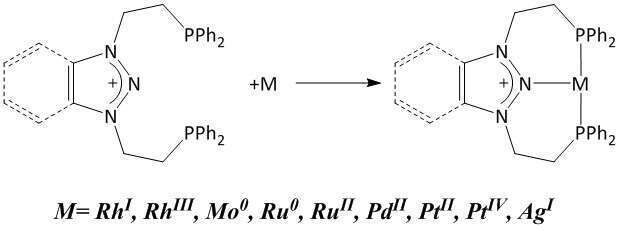
Scheme 1. Pincer type nitrenium ligands coordinate to a variety of late transition metals in different oxidation states.
Nitrenium ligands, contrary to NHC, bear positive charge on the central nitrogen atom. The coordination of those ligands to a transition metal renders the metal center electrophilic. This effect is enhanced when the nitrenium is coordinated to the cationic metal species. Here we present the mono- and di-cationic Ir(I) complexes coordinated to the N-heterocyclic nitrenium cations. Such dicationic (or tricationic) complexes featuring cation-cation L+-M+ interactions are especially attractive for electrophilic catalysis.[2]
The formation of the nitrenium-metal bond is confirmed in solution, by selective 15N-labelling experiments, and/or in the solid state, by X-ray crystallography.
[1] (a) Tulchinsky, Y.; Iron, M. A.; Botoshansky, M.; Gandelman, M. Nature Chem. 2011, 3, 525-531. (b) Tulchinsky, Y.; Kozuch, S.; Saha, P.; Botoshansky, M.; Shimon, L.; Gandelman, M. Chem. Sci. 2014, 5, 1305-1311. (c) Tulchinsky, Y.; Kozuch, S.; Saha, P.; Mauda, A.; Nisnevich, G.; Botoshansky, M.; Shimon, L. J. W.; Gandelman, M. Chem. Eur. J. 2015, 21, 7099−7110.
[2] Unpublished results.

Powered by Eventact EMS